Грипп – наиболее динамичная инфекция, вызывающая эпидемии (когда заболевают 20–30% детей) и периодически – пандемии, наносящие значительный экономический урон [1–4]. Гриппом и гриппоподобными инфекциями дети болеют значительно чаще, чем другими инфекционными болезнями, что подчеркивает их эпидемиологическую значимость [5]. По оценкам специалистов, дети в возрасте до 3 лет относятся к группе повышенного риска по гриппу [6, 7]. Так, по данным И.Г. Маринича и соавт. [4], за период с 1986 по 2008 г. в Российской Федерации заболеваемость гриппом и ОРВИ детей по сравнению с взрослыми была выше в 10 раз, детей дошкольного возраста – в 16 раз.
Вакцинация является основным профилактическим мероприятием в борьбе против гриппа и рекомендована как в России, так и за рубежом всем детям в возрасте от 6 мес [6, 8, 9]. В наибольшей степени в защите против гриппа нуждаются дети с хроническими легочными заболеваниями, болезнями сердца, сахарным диабетом, иммунопатологией, включая ВИЧ-инфекцию [10–14]. Большинство исследователей подтверждают, что вакцинация значительно снижает риск заболевания гриппом. Профилактическая эффективность гриппозных вакцин у привитых детей, в том числе часто болеющих гриппоподобными заболеваниями, подтверждена во многих исследованиях. Доказано, что при своевременной вакцинации можно предотвратить заболевание у 80–90% детей и взрослых. Если же привитые заболевают, болезнь у них протекает легче и без каких-либо осложнений [2, 15, 16].
Учитывая высокую заболеваемость гриппом детей и ключевую роль детских контингентов в распространении инфекции, были высказаны предположения, что массовая вакцинация детей может не только предохранять их от заболевания гриппом, но также влиять на снижение заболеваемости лиц других возрастных групп, не получавших прививки. Например, результаты изучения эффективности вакцинации в Свердловской области доказали, что заболеваемость гриппом и гриппоподобными инфекциями среди детского населения, привитого против гриппа, в 2,5 раза ниже, чем среди их непривитых сверстников.
Целью проведенного исследования явилось определение безопасности, реактогенности и иммуногенности вакцины «Гриппол® плюс» в дозах 0,25 и 0,5 мл у детей в возрасте от 6 до 35 мес включительно.
Материалы и методы
Отечественная гриппозная тривалентная инактивированная полимер-субъединичная вакцина «Гриппол® плюс» (регистрационное удостоверение № ЛСР-006981/08) разработана российской компанией ООО «НПО Петровакс Фарм». Вакцина включает высоко очищенные протективные антигены (гемагглютинин и нейраминидазу), выделенные из актуальных штаммов вирусов гриппа типов А и В, выращенных на куриных эмбрионах производства компании «Эбботт Биолоджикалз Б.В.» (Нидерланды), и водорастворимый иммуноадъювант полиоксидоний [17]. Одна иммунизирующая доза (0,5 мл) содержит по 5 мкг гемагглютинина каждого из 3 вирусов гриппа и 500 мкг полиоксидония; вакцина не содержит консервантов.
Гриппол® плюс активно используется для иммунизации населения Свердловской области и в структуре вакцин ее доля составляет не менее 20%. В эпидемический сезон 2011–2012 гг. её доля в структуре всех вакцин составила 28,2%. Данный препарат, в соответствии с инструкцией, применялся для иммунизации детей с 3 лет [17]. Всего в области этой вакциной было привито 440 тыс. детей, из них 25,8% посещали дошкольные образовательные учреждения, 74,2% – учащиеся 1–11 классов.
В соответствии с утвержденным Протоколом клинического исследования, в течение эпидемических сезонов 2010–2011 и 2011–2012 гг. нами было проведено рандомизированное двойное слепое контролируемое сравнительное исследование в параллельных группах, в которое были включены 140 детей в возрасте от 6 мес до 3 лет.
Включение детей в исследование проводили в соответствии с критериями включения/исключения Протокола. Дети, удовлетворяющие критериям включения, были рандомизированы в 2 группы. В 1-ю вошли 70 детей [36 (51%), мальчиков и 34 (49%) девочки, средний возраст – 18±7 мес], вакцинированных Гриппол® плюс в дозе 0,5 мл, во 2-ю – 70 детей [35 (50%) мальчиков и 35 (50%) девочек, средний возраст – 19±7 мес], вакцинированных в дозе 0,25 мл.
Оценку безопасности и переносимости проводили, анализируя до и после каждой вакцинации клинические и лабораторные показатели, частоту и выраженность местных и общих реакций. Все общие и местные вакцинальные реакции разделяли по общепринятым критериям как слабые, средней степени выраженности и сильные.
Для оценки иммуногенности у привитых проводили забор крови до и через 21–28 дней после 1-й и 2-й вакцинации. Уровень антигемагглютинирующих антител в сыворотках крови определяли в РТГА по общепринятой методике в лаборатории биотехнологии диагностических препаратов НИИ гриппа Минздравсоцразвития России (Санкт-Петербург). Сыворотки исследовали с диагностикумом, приготовленным на основе антигенов эпидемических штаммов вируса гриппа A/California/7/2009 (H1N1)-подобный, A/Perth/16/2009 (H3N2)-подобный и B/Brisbane/60/2008-подобный.
Антигенную активность вакцины оценивали по следующим показателям:
– уровень сероконверсий по сравнению с фоновой сывороткой (число лиц с 4-кратным приростом титров антител);
– уровень серопротекций (определение процента детей с защитным титром антител до и через 3–4 нед после 1-й и 2-й вакцинации);
– кратность прироста антител.
Поскольку специальных критериев оценки иммуногенности гриппозных вакцин у детей нет, использовали критерии для взрослых (СPMP/BWP/214/96): уровень сероконверсий – не менее 40%, серопротекций – не менее 70%, кратность нарастания титров – не менее 2,5.
Полученные данные анализировали с помощью критерия Пирсона (χ²) или точного критерия Фишера. Различия считали достоверными при р<0,05. Вариационный анализ полученных результатов проводили с применением пакета прикладных программ Statistica 6.0. Сравнение количественных показателей (предварительно логарифмированных) иммуногенности выполнено с помощью двухвыборочного t-критерия Стьюдента и парного t-критерия Стьюдента; средняя геометрическая титра антител (СГТА) представлена вместе с 95% доверительным интервалом (ДИ).
Результаты и обсуждение
Клинический анализ крови до и после вакцинации показал, что все значения исследуемых показателей в обеих группах оставались в пределах нормы. Вакцинация Гриппол® плюс в дозе как 0,25, так и 0,5 мл не приводит к патологическим отклонениям от нормы.
Оценка реактогенности вакцины «Гриппол®плюс»
Оценивали местные реакции: боль в месте инъекции при надавливании, покраснение, припухлость. Местные реакции в 1-й группе проявились у 3 (4,3%) детей после 1-й и у 4 (6,6%) после 2-й прививки, во 2-й группе – у 5 (7,4%) детей после 1-й и у 2 (3,1%) после 2-й прививки. Не выявлено достоверных различий в частоте и выраженности местных реакций после 1-й и 2-й вакцинации в обеих группах. Все реакции были слабыми, разрешались самостоятельно в течение 3 дней, не требовали медикаментозного вмешательства.
Оцениваемые общие реакции включали субферильную и фебрильную температуру, недомогание, головную боль, нарушение аппетита, нарушение сна, потливость, насморк, кашель. В 1-й группе общие реакции отмечены у 10 (14,5%) детей после 1-й и у 3 (4,5%) после 2-й прививки, во 2-й группе – у 4 (5,9%) детей после 1-й и у 1 (1,5%) ребенка после 2-й прививки. Кратковременный подъем температуры выше 37,5 °С регистрировали в 1-й группе у 1 ребенка после 1-й и у 1 – после 2-й вакцинации. В большинстве случаев общие реакции не вызывали нарушений самочувствия, длились не более нескольких часов и не требовали дополнительного медицинского вмешательства.
Оценка иммунологической эффективности вакцины «Гриппол®плюс»
По результатам исследований сывороток, взятых до и на 21–28-й день после 1-й вакцинации группы наблюдения были разделены на исходно серонегативных (титр до 1:20) и серонегативных (титры 1:40 и более). Результаты анализа сывороток исходно серонегативных детей после 1-й и 2-й вакцинации, представлены в табл. 1–3.
После 1-й вакцинации в обеих группах показатели 4-кратного прироста титров антител (сероконверсии) и кратность нарастания титров соответствовали международным критериям СРМР только для штамма А/H1N1 (68,3 и 59,3%; 4,6 и 3,1 для доз 0,25 и 0,5 мл соответственно; см. табл. 2). Ответ на 1-ю вакцинацию на 2 других вакцинных штамма A/H3N2 и В был значительно слабее: уровень сероконверсий по штамму A/H3N2 составил 33,3 и 36,6% для доз 0,25 и 0,5 мл, кратность нарастания титров – соответственно 2,2 и 2,2; уровень сероконверсий по штамму В – 19,3 и 17,5% для доз 0,25 и 0,5 мл, кратность нарастания титров – соответственно 1,8 и 1,4.
Показатели серологической защиты (уровень серопротекций) по 3 штаммам вируса гриппа после 1-й вакцинации были низкими.
Следует отметить, что в группе исходно серонегативных детей все 3 показателя иммуногенности (серопротекции, сероконверсии и кратность прироста титров) после 1-й вакцинации дозой 0,25 мл были не ниже, а по кратности нарастания титров к штамму А/H1N1 – достоверно выше (р<0,05) по сравнению с показателями у детей, вакцинированных дозой 0,5 мл.
После двукратной вакцинации Грипполом® плюс во 2-й группе показатели иммуногенности (серопротекция, сероконверсия и кратность нарастания титров антител) соответствовали критериям СРМР для всех вакцинных штаммов (см. табл. 1 и 2). В 1-й группе показатели иммуногенности также, в основном, соответствовали этим критериям, за исключением показателя серопротекции к штамму В (см. табл. 3).
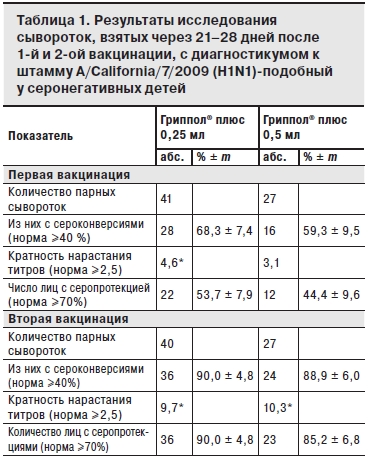
* p<0,01.
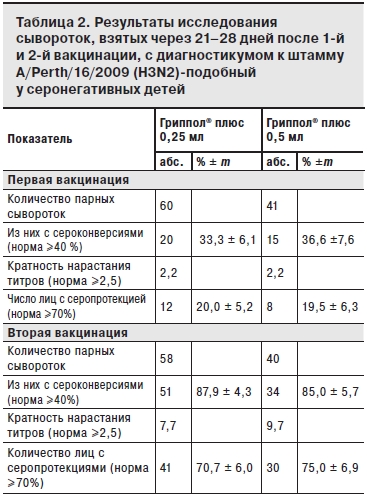
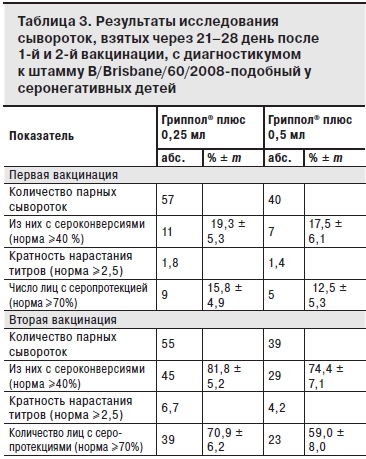
Следует отметить, что после 2-й вакцинации большинство показателей иммуногенности по всем 3 штаммам были выше у детей 2-й группы, однако данные различия статистически недостоверны.
Согласно руководству СРМР по иммуногенности гриппозных вакцин, вакцина считается иммуногенной в том случае, если соответствует, по крайней мере, одному из критериев. Таким образом, на основании анализа результатов проведенного исследования можно рекомендовать для профилактики гриппа двукратную вакцинацию детей в возрасте от 6 мес до 35 мес включительно вакциной «Гриппол® плюс» в дозе 0,25 мл с интервалом в 21 день.
Выводы
В результате проведенных клинических исследований установлено, что вакцина «Гриппол® плюс» для профилактики сезонного гриппа обладает выраженной иммунологической активностью в отношении вирусов гриппа А и В.
Проведенная вакцинация 140 детей в возрасте от 6 до 35 мес гриппозной тривалентной инактивированной полимер-субъединичной вакциной «Гриппол® плюс» в дозах 0,25 и 0,5 мл позволила получить данные, свидетельствующие о безопасности, хорошей переносимости и слабой реактогенности вакцины при двукратном применении. При введении дозы 0,25 мл после 2-й вакцинации жалобы практически отсутствовали.
На основании анализа результатов исследования можно рекомендовать вакцину «Гриппол® плюс» для профилактики гриппа у детей в возрасте от 6 до 35 мес по следующей схеме: двукратная вакцинация дозой 0,25 мл с 3–4-недельным интервалом.


