Хронический гепатит С (ХГС) является глобальной угрозой общественному здравоохранению во всем мире. В России число инфицированных вирусом гепатита С (ВГС) варьирует от 2,2 до 4,9 млн чел., но в 2019 г. лишь 15 662 пациента были обеспечены противовирусной терапией, направленной на полную элиминацию вируса1. При естественном течении у 15–20% пациентов через 20 лет после инфицирования развивается цирроз печени (ЦП), являющийся ежегодно причиной смерти более 1,5 млн чел. В структуре смертности пациентов с хроническими заболеваниями печени одно из первых мест занимает цирроз в исходе гепатита С (ГС) [1]. России принадлежит одно из первых мест в мире по заболеваемости ЦП после Украины, Белоруссии и Литвы. В 2017 г. показатель заболеваемости декомпенсированным ЦП в России составлял 238 на 100 тыс. населения, для компенсированного цирроза он был в несколько раз выше – 2 253 на 100 тыс. населения [2].
Пациенты с ЦП (стадия фиброза F4 по шкале METAVIR), которым противовирусная терапия ГС необходима по жизненным показаниям, в 2017 г. составили 13,1%, со стадией F3 – 11,4%, с начальными стадиями F1 и F2 – 40,1% [3].
Лечение ХГС – актуальная проблема не только для России, но и для всего мирового сообщества. В связи с пандемией новой коронавирусной инфекции COVID- 19 число пациентов, пролеченных от ГС, в 2020 г. по сравнению с 2017 г снизилось на 30%. В условиях пандемии пациенты с ХГС находятся в зоне риска по развитию летального исхода, который у них увеличивается до 30% [4].
Основная цель противовирусной терапии препаратами прямого противовирусного действия – достижение устойчивого вирусологического ответа, который благодаря новым схемам терапии достигает 97% [5–7]. Эрадикация вируса приводит к нормализации уровня ферментов, улучшению функции органа и, как следствие, к регрессу фиброза печеночной ткани, что снижает риск развития ЦП [8–10]. Опубликовано незначительное количество данных о динамике фиброзных изменений в печеночной ткани после проведения курса противовирусной терапии ХГС.
Цель исследования – оценка динамики регресса фиброза печени (ФП) у пациентов с ХГС, получивших курс противовирусной терапии препаратами прямого противовирусного действия.
Материалы и методы
В период с 2018 по 2021 г. под нашим наблюдением находились 59 пациентов с ХГС, различными генотипами вируса и стадиями ФП от F1 до F4 по шкале METAVIR, которые получали противовирусную терапию препаратами прямого противовирусного действия по различным схемам (омбитасвир/паритапревир/ритонавир + дасабувир; глекапревир + пибрентасвир; софосбувир + даклатасвир в сочетании с рибавирином). Длительность терапии варьировала от 8 до 16 нед., определялась согласно клиническим рекомендациям и стандартам лечения. До начала противовирусной терапии всем пациентам была выполнена оценка фиброза и стеатоза печени путем комплексного фибросканирования, данное обследование повторно проведено после окончания курса терапии в период от 0 до 25 мес. у пациентов с устойчивым вирусологическим ответом.
Стадию ФП оценивали с помощью вибрационно контролируемой транзиентной эластометрии, степени стеатоза – путем определения контролируемого параметра затухания на аппарате FibroScan® 530 Compact с ультразвуковым датчиком (М+ и XL+) (Echosens, Франция). Стадию фиброза и степень стеатоза определяли с помощью соответствующих шкал интерпретации [11].
Золотым стандартом определения стадии фиброза является пункционная биопсия печени, которая имеет ряд противопоказаний и осложнений в отличие от комплексного фибросканирования. Достоинство данного метода – возможность динамического наблюдения за морфологическими изменениями в структуре органа, возникающими после полной эрадикации ВГС.
Для градации стадии фиброза используется шкала METAVIR, которая является международным стандартом для оценки состояния печеночной ткани у пациентов с хроническими заболеваниями печени. В данной шкале выделяют 4 стадии, что дает возможность оценить процент соединительной ткани или рубцового замещения на месте гепатоцитов. Стадия F0–F1 соответствует нормальному состоянию структуры печени, на данном этапе можем обнаружить портальный фиброз без вовлечения перегородок (до 7,1 kPa); стадия F2 – начальная, при данном поражении обнаруживается портальный фиброз с вовлечением нескольких перегородок (от 7,2 до 9,4 kPa); стадия F3 соответствует продвинутому фиброзу – в процесс вовлечено множество перегородок, но развития ЦП не наступает (от 9,5 до 12,4 kPa для пациентов с ХГС; для пациентов, снятых с учета по ГС, данный показатель возрастает до 14,4 kPa), стадия F4 – глубокое нарушение архитектоники печени с образованием ложных долек, ЦП (от 12,5–14,5 до 75 kPa соответственно). Степень стеатоза диагностируется с помощью аналогичной шкалы: S0 – нормальный показатель (до 221 dB/m у пациентов с ХГС), замещение составляет от 0–4% нормальной печеночной ткани. При степени S1 (от 222 до 232 dB/m) замещено до 33% печени, при степени S2 (от 233 до 289 dB/m) – 66%, при степени S3 (которая начинается от 290 dB/m) – более 67% [11].
Достоверность различий определяли по критерию χ2 МакНемара. Для подсчета статистического критерия значимости был использован «Калькулятор расчета статистической значимости различий», достоверным считали значения p < 0,05.
Результаты
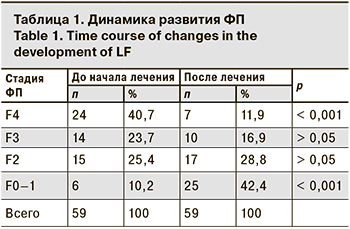
В зависимости от стадии ФП пациенты были разделены на 4 группы. Стадия F4 установлена у 24 (40,7%) пациентов, размах kPa составил от 12,5 до 35,9, средний показатель – 20,0 ± 7,4 kPa. После лечения эластичность печени составила 12,4 kPa ± 7,06 kPa. Обратное развитие фиброза было установлено у 17 (70,8%) пациентов, из них стадия F3 зарегистрирована у 7 (41,2%) пациентов, F2 – также у 7 (41,2%), а у 3 (17,6%) пациентов фиброз уменьшился cо стадии F4 до F0–F1 (χ2 =17; р < 0,001).
Таким образом у 7 больных с F4 со средним показателем 19,4 ± 4,9 kPa до начала лечения выявлено уменьшение до 11,3 ± 1,3 kPa (F3). Эластичность печени улучшилась в 1,6 раза (максимальное снижение – с 24,7 до 13,0 kPa). У 7 чел. упругость печени до начала терапии была 16,7 ± 4,1 kPa, после лечения она составила 8,4 ± 0,4 kPa (F2) (максимальное снижение – с 23,6 до 7,9 kPa), то есть эластичность улучшилась в 2 раза. У 3 пациентов эластичность печени практически нормализовалась – с 15,2 ± 3,1 kPa при старте терапии до 5,28 ± 0,9 kPa (F0–F1) после лечения, произошло снижение показателей практически в 3 раза (наибольшая динамика с 18,8 до 5,4 kPa).
У 7 (29,2%) пациентов стадия фиброза осталась прежней – F4. Необходимо подчеркнуть, что у этих пациентов также выявлена положительная динамика в виде уменьшения kPa: с 20,8 до 18,0; с 35,9 до 13,6; с 30,3 до 28,5; с 21,4 до 14,8; с 41 до 36; с 18,8 до 15 и с 13,5 до 12,8. Средние показатели уменьшились с 25,9 ± 10,0 kPa до 19,8 ± 8,9 kPa (после окончания лечения). Регресс фиброза в этой группе варьировал от 0,7 до 22,3kPa. У 8 (33,3%) пациентов было отмечено увеличение степени стеатоза печеночной ткани.
До старта терапии стадия F3 была у 14 (23,7%) пациентов, разброс показателей составил от 10,0 до 11,4 kPa (в среднем 10,9 ± 0,7 kPa), после лечения эластичность печени составила 7,4 ± 1,5 kPa. Обратное развитие фиброза было установлено у 11 (78,6%) пациентов: стадия F2 диагностирована у 6 (54,5%) чел., F0–F1 – у 5 (45,5%) пациентов. (χ2 = 11; р < 0,001).
У 6 чел. со стадией F3 при старте терапии со средним показателем 10,5 ± 0,7 kPa жесткость печени уменьшилась до 8,2 ± 0,8 kPa (F2) после лечения, показатели улучшились практически в 1,3 раза (максимальный регресс – с 11,1 до 8 kPa). У 5 больных произошла нормализация упругости печени с 11,1 ± 0,5 kPa до начала лечения до 5,7 ± 0,9 kPa (F0–F1), произошло уменьшение жесткости почти в 2 раза (максимально с 11,4 до 6,7 kPa).
У 3 (21,4%) пациентов стадия F3 осталась прежней также с положительной динамикой: показатели kPa уменьшились с 10,8 до 10,0; с 11,9 до 10,5 и с 10,9 до 9,9. Таким образом показатели изменились с 11,2 ± 0,6 kPa до начала лечения до 10,1 ± 0,3 kPa после его окончания. У пациентов отмечена положительная динамика в виде снижения степени стеатоза. Снижение показателя фиброза в данной группе составило от 0,8 до 6,6 kPa.
Стадия F2 до старта терапии зарегистрирована у 15 (25,42%) пациентов, показатель в kPa варьировал от 7,2 до 9,4 (в среднем 8,52 ± 0,8 kPa). После лечения эластичность печени составила 6,0 ± 1,5 kPa. У 11 (73,3%) пациентов диагностирована стадия F0–F1 после курса противовирусной терапии со средним показателем 5,4 ± 1,4 kPa при среднем показателе до лечения 8,4 ± 0,7 kPa, то есть наблюдалось улучшение упругости в 1,6 раза. Максимальное изменение – с 8,7 kPa при старте лечения до 3,5 kPa после его окончания (χ2 = 11; р < 0,001). У этих пациентов не выявлено тенденции к росту степени стеатоза. У 4 (26,7%) больных после завершения терапии сохранилась стадия F2, при этом одному из них исследование было выполнено в день окончания терапии ХГС, и динамика составила от 7,4 до 7,2 kPa. У остальных пациентов снизились показатели в kPa с 9,2 до 7,2; с 9,3 до 7,3. У одного пациента отсутствовала динамика по стадии ФП через 6 мес. восстановительного периода, соответствовавшая 9,4 kPa.
Таким образом, средние значения жесткости печени у пациентов этой группы изменились с 8,7 ± 1,4 kPa при старте терапии до 7,9 ± 1,2 kPa после ее окончания и восстановительного периода. Отмечено увеличение степени стеатоза после завершения курса противовирусной терапии. Обратная динамика ФП составила от 0–0,2 до 5,2 kPa.
Стадия фиброза F0–F1 до старта терапии была у 6 (10,2%) пациентов, размах в kPa составил от 5,4 до 6,1 (в среднемс 5,8 ± 0,2 kPa), после лечения – 4,4 ± 0,6 kPa. ( р = 1). Степень стеатоза снизилась, снижение показателя ФП составило от 0,5 до 2,5 kPa. Динамика ФП представлена в таблице.
Обсуждение
Результаты нашего исследования свидетельствуют о регрессе стадии ФП после завершения противовирусной терапии препаратами прямого противовирусного действия, аналогичные результаты получены и у рядом других авторов. В.Г. Морозов и соавт. [12] получили данные о регрессе ФП у 13 пациентов с переходом одного из них из стадии F4 в стадию F2 с максимальным снижением на 46,7 kPa. В исследованиях Y. Davidov и соавт. [13] и S. Hametner и соавт. [14] зарегистрирован регресс ФП у пациентов в стадию F1 при изначально продвинутых стадиях более чем в 45%. В.В. Макашова и соавт. [15] получили данные о снижении стадии фиброза F4 до F1 у 3 пациентов. Другие исследователи считают, что после эрадикации ВГС и снятия пациентов с учета возможно прогрессирование стадии ФП из-за наличия разнообразной сопутствующей патологии. К числу заболеваний, приводящих к прогрессированию фиброза, с высокой степенью доказательности в первую очередь можно отнести неалкогольную жировую болезнь печени, нарушение микрофлоры кишечника. Доказано также увеличение случаев коморбидной патологии в зависимости от стадии фиброза [15–17]. Динамика показателей степени стеатоза различна, что вызвано алиментарным поведением пациентов, метаболическим синдромом, обусловленным ХГС.
Заключение
Из общей когорты пациентов до старта противовирусной терапии ХГС стадия фиброза F4, соответствующая ЦП, была установлена у 24 (40,7%) пациентов, нормальная архитектоника печени (стадия F0–F1) – только у 6 (10,2%). Выявлена обратная динамика фиброза печеночной ткани после курса терапии и восстановительного периода от 0 до 25 мес. в виде увеличения доли пациентов со стадией F0–F1 в 4 раза – с 10,2 до 42,4%, а доля больных со стадией F4 уменьшилась почти в 4 раза – с 40,7 до 11,9%.
Уменьшение удельного веса больных с выраженной стадией ФП (F3–F4) приводит к снижению частоты возникновения цирроза, что снижает общую смертность населения, страдающего от хронических заболеваний печени, увеличивая продолжительность и улучшая качество жизни пациентов.


