Согласно действующим отечественным и международным рекомендациям, при выборе схем антиретровирусной терапии (АРТ) предпочтение отдают схемам, включающим комбинированные препараты [1–3]. Уменьшение количества принимаемых пациентами таблеток и кратности их приема приводит к повышению удовлетворенности пациентов терапией, приверженности их лечению и, соответственно, эффективности АРТ [4, 5]. В последние годы особую актуальность приобретают схемы, включающие отечественные антиретровирусные препараты. Сравнение режимов АРТ, содержащих отечественный препарат из группы нуклеозидных ингибиторов ВИЧ (НИОТ) фосфазид (Ф-АЗТ) или тенофовира (TDF), показало их сопоставимую вирусологическую и иммунологическую эффективность и безопасность [6]. В 2017 г. на территории Российской Федерации был зарегистрирован первый отечественный препарат из группы ННИОТ элсульфавирин (ESV). Исследование, проведенное в течение 96 нед., показало высокую эффективность и безопасность комбинации ESV и 2 НИОТ [7].
Следующим этапом исследования была оценка эффективности и безопасности схемы АРТ, включавшей Ф-АЗТ и ESV и ламивудин (3TC). Использование в составе режима АРТ Ф-АЗТ может быть альтернативой препаратам TDF и абакавир (АВС), особенно для пациентов, страдающих патологий почек, сердечно-сосудистой системы, снижением минеральной плотности костной ткани, а также при наличии положительного результата теста на HLA B*5701.
Комбинация Ф-АЗТ и ESV и 3ТС была высокоэффективной и безопасной в течение 60 нед. лечения больных ВИЧ-инфекцией, ранее не получавших АРТ [8].
В 2018 г. на территории Российской Федерации зарегистрирован первый отечественный комбинированный препарат, включающий Ф-АЗТ и 3TC – фосфаладин (Ф-АЗТ/3TC), что позволило создать схему АРТ, состоящую исключительно из отечественных препаратов (Ф-АЗТ/3ТС + ESV) с полным производственным циклом в России. Кроме того, включение в схему АРТ комбинированного лекарственного средства позволило уменьшить количество принимаемых пациентом таблеток по сравнению с использованием монопрепаратов Ф-АЗТ и 3ТС с 5 до 3 в сутки.
Целью исследования была оценка эффективности и безопасности схемы АРТ, включавшей фосфаладин, у больных ВИЧ-инфекцией, переведенных со схемы АРТ, содержавшей монопрепараты Ф-АЗТ + 3TC.
В исследование было включено 100 больных ВИЧ-инфекцией, ранее получавших АРТ по схеме: Ф-АЗТ (400 мг 2 раза в сутки) + 3ТС (150 мг 2 раза в сутки) + ESV (20 мг в сутки). Длительность лечения составляла от 24 до 60 нед. 80% больных принимали участие в 1-м этапе исследования [8]. С целью упрощения схемы АРТ всем пациентам вместо 2 отдельных НИОТ (Ф-АЗТ + 3TC) был назначен комбинированный препарат Ф-АЗТ/3TC в дозе 400/150 мг 2 раза в сутки. Все пациенты продолжили прием ESV в дозе 20 мг 1 раз в сутки.
Исходные характеристики пациентов представлены в табл. 1.
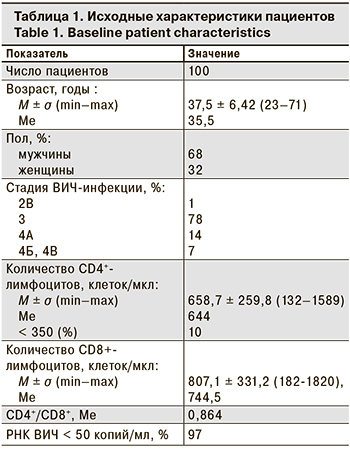
Медиана (Ме) массы тела пациентов составляла 70 кг.
Среди вторичных заболеваний преобладали кандидоз слизистых оболочек ротовой полости, герпетические инфекции (Herpes simplex, Herpes zoster), рецидивирующие респираторные инфекции и снижение массы тела (у 2 пациентов более чем на 10%). У 3 пациентов была диагностирована пневмоцистная пневмония, у 2 – туберкулез легких, по 1 пациенту страдали саркомой Капоши, манифестной цитомегаловирусной инфекцией, кандидозом слизистых оболочек пищевода. У всех участников исследования вторичные заболевания были в анамнезе.
На момент начала 2-го этапа исследования у 97 из 100 больных ВИЧ-инфекцией уровень РНК ВИЧ был < 50 копий/мл. У 3 пациентов он составлял 98, 113 и 17 483 копии/мл, что, вероятно, было связано с неполной приверженностью их лечению, поскольку к 24-й неделе терапии после переключения у этих пациентов уровень РНК ВИЧ был < 50 копий/мл.
На момент переключения на комбинированный препарат 96 из 100 пациентов никаких жалоб не предъявляли. Двух пациентов беспокоили слабость, повышенная утомляемость, периодические подъемы температуры тела до субфибрильных значений, 1 – боли в суставах и 1 – рецидив Herpes simplex genitals. У этих 4 больных количество CD4+-лимфоцитов составляло 531–1021 клетку/мкл, а уровень РНК ВИЧ – < 50 копий/мл.
Сопутствующие заболевания органов желудочно-кишечного тракта и печени (ХГС, хронический гастрит, хронический панкреатит, язвенную болезнь 12-перстной кишки) имели 10 пациентов, сердечно-сосудистой системы (гипертоническую болезнь, ИБС) – 4, сахарный диабет – 3 (2 – 2-го типа, у 1 заболевание 1-го типа установлено в процессе исследования), болезни почек (хронический гломерулонефрит, пиелонефрит) – 2. До начала исследования и далее через 12, 24, 36 и 48 нед. методом проточной цитометрии определяли количество CD4+- и CD8+-лимфоцитов, методом ПЦР – уровень РНК ВИЧ (чувствительность теста – 50 копий/мл).
Безопасность схемы АРТ оценивали по частоте развития НЯ различной степени тяжести по данным субъективных жалоб, физикального осмотра, жизненных показателей, лабораторных исследований. Параметры анализа периферической крови и биохимического анализа крови исследовали до переключения на комбинированный препарат, через 12, 24, 36 и 48 нед. терапии. Показатели обмена липидов крови [общий холестерин (ОХ), липопротеиды высокой плотности (ЛПВП), липопротеиды низкой плотности (ЛПНП), триглицериды] определяли до начала исследования, через 24 и 48 нед. лечения. Также оценивали индекс атерогенности (ОХ/ЛПВП).
Приверженность лечению оценивали по количеству принятых пациентом доз исследуемого препарата, по результатам эффективности, а также на основании результатов заполнения упрощенного вопросника для оценки точности соблюдения режима приема препарата (Simplified Medication Adherence Questionnaire – SMAQ).
Внутригрупповые изменения параметра оценивали с помощью t-теста Стьюдента для нормально распределенных данных или знакового критерия Вилкоксона (Манна–Уитни) для данных, не имеющих нормального распределения. В качестве теста на нормальность распределения использовали тест Шапиро–Вилка. Результаты обрабатывали с помощью компьютерной программы Biostat.
Результаты
В течение 48 нед. после переключения на комбинированный препарат Ф-АЗТ/3TC неопределяемый уровень РНК ВИЧ регистрировали у 97,5–99% больных (см. рисунок). У отдельных пациентов в разные недели наблюдения отмечали уровень РНК ВИЧ > 50 копий/ мл, при этом ни в одном случае не выявляли РНК ВИЧ > 1000 копий в 2 последующих образцах плазмы, полученных с интервалом 12 нед. Через 48 нед. АРТ только у 2 пациентов уровень РНК ВИЧ был > 50 копий/мл (644 и 7535 копий/мл). 99–100% пациентов имели высокий уровень приверженности лечению (принимали > 95% назначенных доз препаратов). Только у 1 пациента через 24 нед. АРТ приверженность составляла 92%, а у 1 через 48 нед. – 89% (пропуск приема лекарств составил 10 дней). Вместе с тем у обоих больных уровень РНК ВИЧ был < 50 копий/мл.
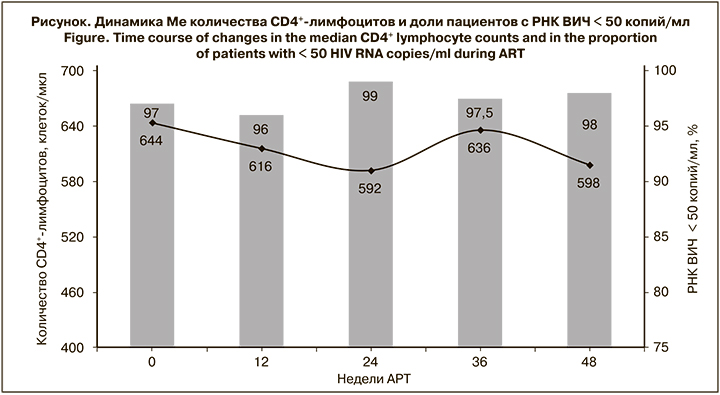
В процессе лечения регистрировали несущественные колебания Ме количества CD4+-лимфоцитов от 592 до 644 клеток/мкл (p > 0,05). Также отмечали колебания Ме иммунорегуляторного индекса от 0,864 до изменения схемы АРТ до 0,800 через 24 нед. лечения и до 0,877 через 48 нед. В то же время на момент изменения схемы АРТ количество CD4+-лимфоцитов < 350 клеток/ мкл было у 10 больных, а через 36-48 нед. – у 6–7. За период наблюдения ни у одного пациента не обнаружили симптомов вторичных заболеваний, что свидетельствовало об отсутствии клинической прогрессии ВИЧ- инфекции.
Необходимо отметить, что в течение 48 нед. терапии 17 пациентов перенесли коронавирусную инфекцию, 18 – ОРВИ неуточненной этиологии, а 3 пациента отмечали лихорадочную реакцию после вакцинации Sputnik V. Кроме того, пациенты получали сопутствующую терапию (фавипиравир, азитромицин, арбидол, цефалоспорины и др.), которая, как и острые вирусные инфекции, могла оказать влияние на количество CD4+-лимфоцитов. Ранее нами был описан клинический случай бессимптомного течения коронавирусной инфекции у больного ВИЧ-инфекцией со снижением количества CD4+-лимфоцитов на 200 клеток/мкл при неопределяемом уровне РНК ВИЧ (< 50 копий/мл) [9].
Переносимость схемы АРТ была хорошей: 94–96% пациентов никаких жалоб на момент осмотра не предъявляли. НЯ, связанных с терапией, зарегистрировано не было. Единичных пациентов беспокоили слабость, повышенная утомляемость, периодические головные боли и смена настроения. НЯ были легкой степени и в большинстве случаев не требовали коррекции схемы АРТ или назначения дополнительной терапии. После 12–36 нед. после переключения на комбинированный препарат 3 пациента отметили увеличение массы тела на 6–8 кг в течение последнего года терапии. Если до изменения схемы АРТ средняя масса тела больных составляла 73,6 ± 14,9 кг (Ме – 70 кг), то через 48 нед. она изменилась не существенно и составила 74,7 ± 14,2 кг (Ме – 72 кг; p > 0.05). Таким образом, увеличение массы тела за 48 нед. исследования составило по средним значениям +1,1 кг, по Ме – 2 кг.
В процессе исследования не отмечено существенных колебаний Ме уровня Hb (144,2–148,5 г/л), ни у одного больного уровень Hb не был < 100 г/л. У 2,0–7,6% пациентов регистрировали снижение количества эритроцитов < 3,0 х 1012/л, при этом Ме количества эритроцитов изменилась минимально (3,69–3,79 х 1012/л; табл. 2). В течение 48 нед. терапии не наблюдали изменений количества тромбоцитов и лейкоцитов в анализе периферической крови. Ни у одного пациента не выявили тромбоцитопению или лейкопению.
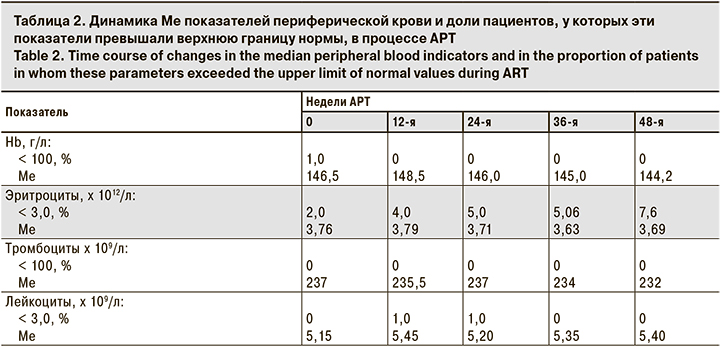
В табл. 3 представлена динамика Ме биохимических показателей и доли пациентов, у которых эти показатели превышали верхнюю границу нормы (глюкоза, креатинин) или были повышены до 2–4-й степени токсичности (АлАТ, ГГТ). Существенных колебаний уровней креатинина, глюкозы и АлАТ в процессе лечения зарегистрировано не было. Повышение уровня глюкозы у 5–11% пациентов, как правило, было минимальным, за исключением 3 пациентов, которые страдали сахарным диабетом. Отмечено некоторое увеличение активности ГГТ, а также доли пациентов (3,85–8,0%; p > 0,05), у которых показатели были повышены (до 2–4-й степени токсичности), но в большинстве эти отклонения не имели статистической достоверности. При этом ни у одного пациента не было клинических проявлений панкреатита и существенного повышения уровня липазы крови.
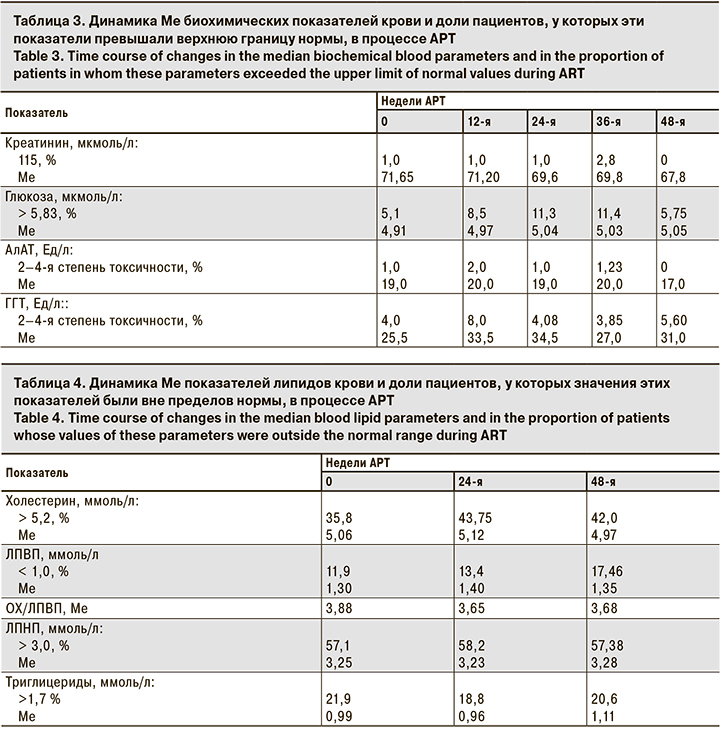
В течение исследования показатели обмена липидов крови изменялись не существенно: Ме содержания общего холестерина составила 5,06–4,97 ммоль/л (p > 0,05), ЛПВП – 1,3–1,35 ммоль/л, ЛПНП – 3,25–3,28 ммоль/л, триглицеридов – 0,99–1,11 ммоль/л (p > 0,05; табл. 4). Отмечено некоторое увеличение доли пациентов с повышенным уровнем общего холестерина с 35,8 до 42% (p > 0,05), которое практически не отразилось на значениях индекса атерогенности (ОХ/ЛПВП) – 3,88–3,68 (p > 0,05). Для коррекции изменений уровней липидов крови пациентам не потребовалось назначения липидоснижающих препаратов.
Обсуждение
Переключение пациентов, получавших эффективную схему АРТ, включавшую 3 отдельных антиретровирусных препарата (Ф-АЗТ + 3ТС + ESV), на сочетание комбинированного препарата Ф-АЗТ/3ТС и ESV позволило сократить количество таблеток, принимаемых пациентом в течение суток, с 5 до 3.
Проведенная нами оценка качества жизни пациентов не выявила сколько-нибудь значимого его снижения. Удовлетворенность АРТ пациенты оценили на 8,45 балла из 10, при этом 85,4% из них дали оценку выше 5 баллов. Удовлетворенность выраженностью побочных эффектов составила 8,85 балла из 10. 77,1% пациентов отметили удобство приема терапии выше 5 баллов [10].
Уменьшение количества принимаемых таблеток позволило 99–100% пациентов поддерживать высокий уровень приверженности лечению в течение 2-го года терапии, в результате чего у 97,5–99% больных сохранялся неопределяемый уровень РНК ВИЧ. У 2 пациентов, несмотря на неполную приверженность лечению (92 и 89%), уровень РНК ВИЧ был < 50 копий/мл, что свидетельствует о достаточно высокой противовирусной активности и хорошем генетическом барьере данного терапевтического режима.
При оценке эффективности схемы TDF + 3TC + ESV у 5,3% пациентов был установлен вирусологический неуспех терапии, связанный с резистентностью вируса к применяемым препаратам. У всех пациентов была обнаружена устойчивость вируса к 3ТС (мутации M184I/V). Только у 1 больного со средним уровнем приверженности терапии была выявлена комбинация мутаций, снижающая чувствительность к ESV (мутации V106I, F227C + E138K), и у 4 пациентов выявлены мутации, которые способны снижать чувствительность к препарату в комбинации с другими мутациями [11].
В процессе исследования Ме количества CD4+-лимфоцитов колебалась в пределах 592–644 клеток/ мкл, что, возможно, было обусловлено заболеванием коронавирусной инфекцией ряда пациентов или вакцинацией от COVID-19. Ни у одного пациента не было симптомов вторичных заболеваний, что свидетельствует об отсутствии клинической прогрессии ВИЧ-инфекции.
Переносимость схемы АРТ была хорошей, никаких НЯ, связанных с лечением, зарегистрировано не было, а у 94–96% пациентов не было отмечено вообще никаких НЯ. Ни у одного пациента схема терапии не была отменена или изменена из-за развития НЯ. Показатели периферической крови и параметры биохимического анализа крови изменялись не существенно. Не отмечено снижения содержания Hb крови < 100 г/л. Небольшое повышение уровня активности ГГТ у 4–8% больных не сопровождалось повышением уровня липазы крови и какой-либо симптоматикой панкреатита. Изменения показателей липидов крови носили разнонаправленный характер, при этом Ме ОХ/ЛПВП изменялась не существенно.
Заключение
Таким образом, результаты исследования свидетельствуют о высокой эффективности и хорошей безопасности отечественной схемы АРТ, включавшей комбинированный препарат фосфаладин (Ф-АЗТ/3ТС) и ESV. Данная схема АРТ может быть рекомендована больным ВИЧ-инфекцией в качестве схемы первой линии терапии.


