Коронавирусная инфекция (COVID-19), вызываемая вирусом SARS-CoV-2, впервые выявленная в декабре 2019 г., характеризуется быстрым распространением и в большой доле случаев может приводить к летальному исходу, затрагивая дыхательную и сердечно-сосудистую системы [1]. К моменту начала исследования по состоянию на 1 января 2021 г. в мире зарегистрировано более 80 млн случаев заболевания COVID-19 и почти 1,8 млн смертей. Пандемия привела к снижению доступа к услугам по профилактике и лечению ВИЧ-инфекции, что явилось угрозой для сохранения контроля над эпидемией ВИЧ [2].
Описанная ситуация обусловила потребность в эффективных и безопасных вакцинах для снижения скорости распространения, минимизации осложнений, психологических и экономических издержек во всем мире [3]. Первой зарегистрированной вакциной в мире явилась отечественная векторная вакцина Спутник V (Гам-КОВИД-Вак, регистрационное удостоверение ЛП-006395 от 11.08.2020), которая прошла все 3 фазы клинических исследований, показала свою высокую эффективность, ее успешно начала широко использовать с января 2021 г. [4]. Эта 2-компонентная вакцина состоит из рекомбинантных, неспособных к репликации векторов аденовируса человека серотипов 26 и 5, которые проникают в клетки человека, обеспечивая экспрессию шиповидного белка без размножения вируса, что затем вызывает иммунный ответ, защищающий от COVID-19. Впоследствии было зарегистрировано еще несколько вакцин с аналогичным действием производства США (Johnson & Johnson и Janssen-Cilag International), Великобритании (AstraZeneca) и Китая (CanSino Biologics), но в их составе содержится лишь 1 тип аденовируса.
Второй тип разработанных вакцин содержит очищенную матричную РНК (мРНК) вируса, внедренную в липидные частицы, что позволяет доставлять ее в клетки, обеспечивая экспрессию спайкового белка SARS-CoV-2. В результате вызывается иммунный ответ на шиповидный белок, который защищает от COVID-19. Примеры таких вакцин – Pfizer-BioNTech и Moderna.
Третий тип – пептидные вакцины, которые содержат искусственно синтезированные короткие фрагменты вирусного белка SARS-CoV-2 (ЭпиВакКорона, Россия; Anhui Zhifei Longcom Biopharmaceutical, Китай).
Четвертый тип – химически инактивированные цельновирусные вакцины (КовиВак, Россия; CoronaVac и BBIBP-CorV, Китай). Поскольку ни один из типов вакцин не содержит живого вируса, развитие заболевания в результате введения вакцины невозможно.
На заре появления вакцин от COVID-19 пациентов с иммунодефицитными состояниями исключали из клинических исследований, что было обусловлено опасениями по поводу эффективности вакцины и потенциальных последствий среди этой группы пациентов. В 2021 г. консультативный совет по исследованиям СПИДа Национального института здравоохранения DHHS опубликовал временное руководство для пациентов с COVID-19 и ВИЧ [5]. В настоящее время большинство исследователей поддерживают рекомендацию вакцинации всех людей, живущих с ВИЧ (ЛЖВ), независимо от количества CD4+-лимфоцитов или вирусной нагрузки (ВН), поскольку потенциальные преимущества перевешивают потенциальные риски [6]. Специалисты отмечают, что пациенты с ВИЧ на стабильной антиретровирусной терапии (АРТ) исторически хорошо реагируют на другие вакцины, применяемые в клинической практике, но информации о безопасности и эффективности вакцин против COVID-19 для этой группы населения недостаточно. Впоследствии, хотя ЛЖВ и были включены в исследования, опубликованные данные об этой популяции ограничены. Так, эффективность и безопасность вакцины Pfizer изучали у пациентов со стабильной ВИЧ-инфекцией (количество CD4+-лимфоцитов > 200 клеток/мкл, неопределяемая ВН, получение стабильной АРТ в течение как минимум 6 мес. до включения в исследование). ЛЖВ составили 0,3% исследуемой популяции (121 пациент) и не были включены в первичный анализ [7]. В исследование вакцины Moderna были включены 159 пациентов со стабильной ВИЧ-инфекцией (количество CD4+-лимфоцитов > 350 клеток/мкл, неопределяемая ВН в течение 12 мес.), что составило 0,06% изучаемой группы [8]. Согласно отчету FDA, опубликованному в декабре 2020 г., ни у одного ЛЖВ не развился COVID-19 в течение 14 дней после введения второй дозы вакцины Moderna и в среднем в течение 2 мес. после этого [9]. Самое большое число ЛЖВ участвовали в исследовании вакцины Janssen (1218 пациентов, 2,8% участников исследования), но они также не вошли в первичный анализ [10]. Китайские исследователи изучали эффективность и безопасность четырех собственных вакцин разных типов (аденовекторных и инактивированных) у 383 ЛЖВ, получающих стабильную АРТ. Диапазон наблюдений составил 6 мес. После вакцинации отмечен небольшой рост иммунного статуса и сохранялась неопределяемая ВН, что подтвердило тезис о сохранении контроля за ВИЧ-инфекцией после вакцинации [11].
Центральное место в достижении высокого уровня охвата вакцинацией занимает преодоление нерешительности в отношении вакцин. По данным французских авторов [12], освещение преимуществ коллективного иммунитета и уверенность в безопасности предлагаемой вакцины против COVID-19 важны для минимизации сомнений в отношении прививок у ЛЖВ.
При анализе литературных источников мы не встретили научных работ, в которых были отслежены случаи заражения коронавирусом больных ВИЧ-инфекцией после вакцинации, проанализирована доля таких пациентов, степень тяжести и исходы заболевания. Целью исследования стал–сравнительный анализ клинико-лабораторных исходов после курса вакцинации от инфекции, вызываемой SARS-CoV-2, и при ее отсутствии у ВИЧ-инфицированных пациентов, находящихся на стабильной АРТ.
Материалы и методы
Мы провели проспективно-ретроспективное нерандомизированное наблюдательное сравнительное исследование с января 2021 г. по июнь 2022 г. среди пациентов, находящихся на диспансерном наблюдении в специализированном научно-исследовательском отделе эпидемиологии и профилактики СПИД (СНИОЭП СПИД) Центрального НИИ эпидемиологии и Университетской клинике H-clinic.
В исследовании принял участие 151 пациент, получавший стабильную АРТ (РНК ВИЧ≤50 копий/мл) минимум 12 мес. на момент включения. 51,6% пациентов принимали схемы АРТ на основе ненуклеозидных ингибиторов обратной транскриптазы (элсульфавирин – 22,5%, эфавиренз – 13,9%, рилпивирин – 7,9%, доравирин – 7,3%), еще 39,8% – на основе ингибиторов интегразы (долутегравир – 33,8%, биктегравир – 4%, ралтегравир – 2%), у оставшиеся 9,2% пациентов схемы включали ингибиторы протеазы (дарунавир, атазанавир, лопинавир). Тенофовир или тенофовира алафенамид входили в состав 41,1% режимов АРТ, фосфазид – 21,2%, абакавир – 6%. Кроме того, двойные схемы (как правило, долутегравир и ламивудин) получали 30,5% пациентов.
1-ю группу составили 100 пациентов (83% мужчин и 17% женщин), которые при посещении врача сообщили, что прошли полный курс вакцинации от COVID- 19, 2-ю (контрольную) группу – 51 ЛЖВ (66,7% мужчин и 33,3% женщин), которые не прививались по разным причинам. Исходно группы не имели существенных различий. Средний возраст по медиане (Ме) составил 40 ± 7,5 года в 1-й группе и 38 ± 6,7 – во 2-й. Стадия 3 диагностирована у подавляющего большинства пациентов (84 и 84,3% в 1 и 2-й группах соответственно), стадия 4А – у 12 и 13,7% больных, стадия 4Б – у 2% пациентов каждой группы, стадия 4В – у 2 пациентов 1-й группы. У всех больных на стадии 4 в анамнезе были вторичные заболевания. Сопутствующую патологию имели 33 и 29,4% участников исследования. Из инфекционных заболеваний регистрировали сифилис, хронические гепатиты В и С, хроническую герпетическую инфекцию. У остальных пациентов диагностировали хронические неинфекционные заболевания желудочно-кишечного тракта, сердечно-сосудистой системы, почек, простаты, миому матки, сахарный диабет, аллергические заболевания.
Оценивали течение ВИЧ-инфекции, эффективность АРТ, наличие вторичных и сопутствующих заболеваний. У пациентов 1-й группы указанные показатели регистрировали до вакцинации (1-я контрольная точка), а также после завершения курса вакцинации (2-я контрольная точка). Интервал между контрольными точками составил 3–6 мес. У пациентов 2-й группы показатели оценивали в тех же временных интервалах (1-я и 2-я контрольная точка). Кроме того, у больных обеих групп фиксировали возникновение коронавирусной инфекции в течение исследуемого периода, степень ее тяжести и госпитализации по причине COVID-19. Данные по заболеванию собирали из выписок, результатов лабораторного обследования, предоставленных пациентами, и результатов анкетирования.
Безопасность и переносимость вакцин против COVID-19 оценивали по местным (боль, отек, покраснение) и системным (лихорадка, утомляемость, мышечная и суставная боль, головная боль) нежелательным явлениям. Причинно-следственная связь между нежелательными явлениями и вакцинацией была установлена исследователями.
Статистическую обработку результатов производили параметрическими и непараметрическими методами с использованием программы Biostat.
Результаты и обсуждение
Пациентам 1-й группы полный курс вакцинации был завершен вакцинами Спутник V – 81%, КовиВак – 11%, ЭпиВакКорона – 5%, Pfizer – 3%.
По данным анкетирования, в 1-й группе 56% пациентов приняли решение о вакцинации самостоятельно, 33% – по рекомендации лечащего врача, 11% – по требованию работодателя. В лечебном учреждении (поликлинике, медицинском центре) вакцинировались 83% больных, 17% прививались в торговых центрах. Причинами отказа от вакцинации во 2-й группе были опасения по поводу вакцинации любой или конкретной вакциной у 62,7% пациентов, перенесенная ранее коронавирусная инфекция – у 13,7%, «отсутствие времени» – у 13,7%, медицинские противопоказания – у 9,8%. Большинство воздержавшихся от прививки в связи со страхом побочных эффектов не получили адекватной информации от врачей («врач не советовал прививаться», «еще не посещал врача») или получили противоречивую информацию. Результаты китайского исследования [11] показали, что пациенты, с которыми врачи обсуждают вопросы вакцинации и рекомендуют вакцину, с большей вероятностью будут привиты. Полученные нами данные согласуются с результатами предыдущего исследования, проведенного в нашем центре, где подчеркивалась роль врачей в информировании и поощрении принятия решения пациентами о вакцинации [13].
Сообщили по крайней мере об одной нежелательной реакции в течение 21 дня после обеих доз вакцины 20% пациентов. После 1-го компонента симптоматика наблюдалась у 62% больных, после 2-го – у 24%.
Самой частой местной побочной реакцией была боль в месте инъекции (в 16–28% случаев; рис. 1), которая сохранялась в течение 1–3 сут.
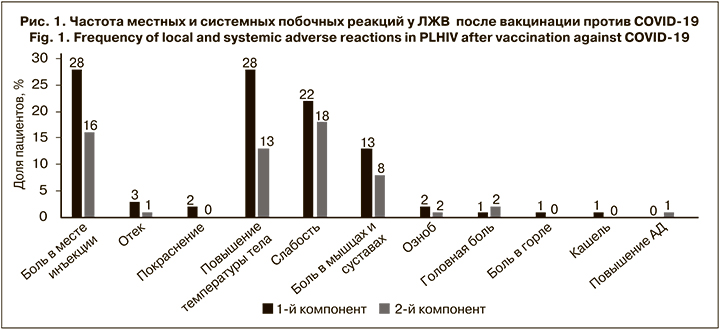
Наиболее распространенными системными побочными реакциями были лихорадка (13–28%), слабость и утомляемость (18–22%), а также ломота в мышцах и суставах (8–13%). Длительность лихорадки и костно-мышечных симптомов не превышала 3 сут., а повышенную утомляемость некоторые пациенты отмечали до 7 дней после вакцинации. Лишь у 5 (5%) больных после вакцинации 1-м компонентом вакцины лихорадка превышала 38 °C. Все нежелательные реакции были легкими (1-й или 2-й степени тяжести) и проходили самостоятельно. Отметим, что 34% пациентов не имели нежелательных реакций ни после 1-й, ни после 2-й дозы препаратов. Полученные результаты свидетельствуют о том, что вакцины против COVID- 19 имеют у ЛЖВ хороший профиль безопасности, не отличающийся от основного населения.
Чтобы изучить влияние вакцины против COVID-19 на эффективность АРТ у ЛЖВ, мы сравнили количество CD4+-лимфоцитов и РНК ВИЧ до вакцинации и спустя 3–6 мес. (сроки зависели от кратности визитов пациентов в клинику). Во 2-й группе тестирование на иммунный статус и ВН проводили в тех же контрольных точках. Среднее количество CD4+-лимфоцитов по Ме у вакцинированных составляло 622 ± 235 клеток/ мкл до вакцинации и увеличилось до 646 ± 223 клеток/мл после нее (p = 0,037; рис. 2), в то время как у непривитых ЛЖВ этот показатель не изменился (599 ± 228 клеток/мкл против 598 ± 221 клеток/ мкл; p = 0,853). Такая же тенденция была получена для процентного соотношения CD4+-лимфоцитов. В 1-й группе после курса вакцинации доля увеличилась с 32 ± 7,6% до 33 ± 8,0% (p < 0,05), тогда как во 2-й группе осталась прежней (35 ± 8,4% против 35 ± 8,5%; p > 0,5).

Исходно в группах не было пациентов с низким иммунным статусом (< 200 клеток/мкл). Вакцинация не привела к снижению количества CD4+-лимфоцитов, а доля больных с высоким иммунным статусом (> 500 клеток/мкл) в 1-й группе после вакцинации даже несколько возросла, в то время как во 2-й группе за тот же период не изменилась, но достоверной разницы не получено (см. таблицу).
В некоторых предыдущих исследованиях говорилось о снижении иммунного статуса после вакцинации, но в них были использованы мРНК-вакцины. Так, в работе израильских авторов [14], где применялась вакцина Pfizer, регистрировали статистически значимое снижение количества CD4+-клеток между исходным и измеренным после 1-й и 2-й вакцинации, а также через 4 мес. после завершения курса. При исследовании векторных и инактивированных вакцин были получены результаты, сходные с нашими. Например, в исследовании II–III фазы по применению векторной вакцины ChAdOx1 nCoV-19 у 54 ЛЖВ в Великобритании на 14-й день после 1-й дозы зафиксировано увеличение количества как CD4+-, так и CD8+-лимфоцитов, но этот эффект был кратковременным [15]. Китайские авторы [11] также опубликовали результаты исследования, в котором применялись инактивированные и векторные вакцины, был получен рост иммунного статуса у пациентов после вакцинации. Основываясь на предыдущих исследованиях других вакцин у больных ВИЧ-инфекцией, можно предположить, что пролиферация CD4+-клеток может быть связана с образованием вирус-специфических нейтрализующих антител [16]. Однако точный механизм этого явления требует дальнейшего изучения.
Повышения уровня ВН (> 50 копий/мл) не было зарегистрировано ни в одном случае, что свидетельствует об отсутствии негативного влияния исследуемых вакцин против COVID-19 на эффективность АРТ.
В 1-й группе в течение 3–6 мес. после последней дозы заболели COVID-19 10 пациентов, во 2-й – 19 (10% против 37,3%; p < 0,01), а среднетяжелое или тяжелое течение коронавирусной инфекции (сочеталось с вирусной пневмонией с поражением более 25% легких, требовалась госпитализация) регистрировали у 1 пациента 1-й группы и у 7 пациентов 2-й группы (1% против 13,7%; p < 0,01).
Таким образом, достоверные шансы заболеть COVID- 19 без вакцинации у пациентов с ВИЧ- инфекцией возрастают в 4,8 раза (ОШ 4,8; 95% ДИ 3,95–5,65), а тяжело заболеть – в 15,8 раза (ОШ 15,8; 95% ДИ 13,7–17,9) по сравнению с вакцинированными ЛЖВ.
Из всех заболевших 51,7% участников имели различные сопутствующие заболевания, а у тех, кто не заболел в исследуемые сроки, сопутствующая патология была диагностирована лишь в 22,3% случаев (p < 0,01). Подтвержденные сопутствующие заболевания или возраст старше 55 лет среди тяжело заболевших имели 85,7% участников исследования. Схемы АРТ и вид вакцины не оказали влияния на заражение коронавирусом и течение заболевания. Полученные данные согласуются с результатами предыдущих исследований [11].
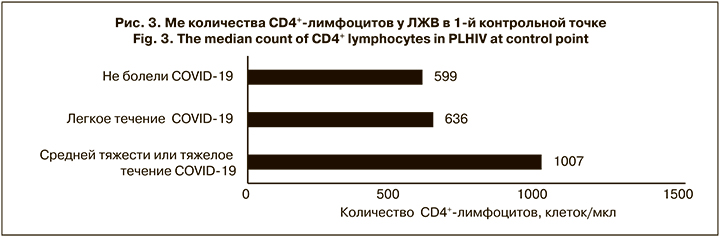
Интересным оказался тот факт, что ЛЖВ, болевшие коронавирусной инфекцией в среднетяжелой или тяжелой форме, имели существенно более высокий иммунный статус (Ме 1007 ± 324 клеток/мкл; рис. 3), чем пациенты, переносившие заболевание легко или не заболевшие. Разница в количестве CD4+-лимфоцитов у больных с легким течением COVID-19 и неболевших была незначительной, но достоверной (p < 0,05), и выражала общую тенденцию. Возможно, основной причиной более тяжелого течения является гипериммунный ответ. Так, некоторые авторы [17, 18] указывают на то, что структурные и функциональные изменения в Т-хелперах, полученные в результате репликации ВИЧ, и последующее нарушение взаимодействия между T-хелперами и В-лимфоцитами способствуют снижению иммунного ответа. У изученной нами группы пациентов, напротив, репликация ВИЧ не только была длительно подавлена, но и произошло полное восстановление пула CD4+-лимфоцитов. Однако этот феномен и наше предположение требуют дальнейших дополнительных исследований с включением большего числа участников.
В подгруппе неболевших, а также ЛЖВ, перенесших заболевание в легкой форме (включая вакцинированных и невакцинированных), мы регистрировали небольшое повышение иммунного статуса во 2-й контрольной точке: с 599 ± 222 до 618 ± 207 клеток/ мкл (p = 0,064) и с 636 ± 207 до 649 ± 227 клеток/ мкл (p = 0,041) соответственно. Напротив, в подгруппе ЛЖВ, перенесших COVID-19 тяжело, существенно уменьшилось абсолютное и относительное количество CD4+-лимфоцитов с 1007 ± 324 до 847 ± 343 клеток/ мкл (p = 0,0038) и на 3% по Ме, что говорит о более выраженном воздействии на иммунную систему коронавируса при тяжелом течении болезни.
Ретроспективный анализ показал, что все ВИЧ- инфицированные 1-й группы, которые заболели, вакцинацию перенесли с полным отсутствием или минимальными побочными реакциями. Лихорадку, не превышавшую 37,6 °С и длившуюся не более 1 сут., отметили только 2 пациента после инъекции 1-м компонентом. Еще несколько больных после 1-й прививки жаловались на дискомфорт в месте инъекции и небольшую слабость. После применения 2-го компонента вакцины нежелательные реакции не были отмечены ни у одного пациента.
Заключение
Анализ нежелательных реакций показал, что вакцины против COVID-19 имеют хороший профиль безопасности у ЛЖВ. Побочные реакции были легкими и не потребовали дополнительных медицинских вмешательств. Лишь 20% привитых сообщили о каких-либо симптомах после инъекции обоими компонентами вакцин. Неожиданных проблем с безопасностью и переносимостью обнаружено не было, а наблюдаемый профиль побочных реакций соответствовал ранее сообщавшемуся для этих типов вакцин. Основными нежелательными явлениями были повышение температуры тела, утомляемость и боль в месте инъекции. У 34% пациентов не было отмечено побочных реакций ни на один компонент вакцин.
Вакцинация не только не ухудшала течение ВИЧ- инфекции и не снижала эффективность АРТ, но и ассоциировалась с увеличением абсолютного и относительного количества CD4+-лимфоцитов, а ВН продолжала оставаться на неопределяемом уровне.
Эффективность вакцинации ЛЖВ от COVID-19 была подтверждена нами в реальной клинической практике. Шансы заболеть COVID-19 без полного курса вакцинации у ЛЖВ были выше в 4,8 раза, а тяжело заболеть – в 15,8 раза по сравнению с непривитыми больными этой категории. При этом острые и хронические сопутствующие заболевания увеличивали риск заражения коронавирусной инфекцией.
Все привитые и впоследствии заболевшие участники исследования переносили вакцинацию с минимальными и краткосрочными нежелательными реакциями на 1-й компонент вакцины, что может свидетельствовать о слабой реакции организма на введение вакцины и, соответственно, недостаточном уровне выработки антител.
Было обнаружено, что непривитые больные ВИЧ-инфекцией, у которых коронавирусная инфекция протекала в среднетяжелой или тяжелой форме, имели существенно более высокий иммунный статус (около 1000 клеток/мкл), чем пациенты с ВИЧ, переносившие заболевание легко или незаболевшие, что вероятно, связано с гипериммунным ответом.
Наше исследование имеет несколько ограничений. Дизайн был наблюдательным с небольшим размером выборки. Имело место смещение выборки – большинство участников состояли на диспансерном наблюдении длительное время и были привержены АРТ, поэтому результаты нельзя экстраполировать на всю популяцию ЛЖВ. Данные о побочных реакциях после вакцинации собирались ретроспективно методом анкетирования, поэтому нуждаются в проверке в крупных проспективных исследованиях.
Необходимы дальнейшие исследования для оценки эффективности вакцин от COVID-19 у ЛЖВ в отношении защиты от тяжелого течения заболевания, госпитализации или смерти в этой популяции. В настоящее время неизвестно, уменьшает ли низкий уровень антител клиническую эффективность. Кроме того, потребуются длительные исследования для определения потенциальной потребности в бустерных дозах вакцины.


