Важное значение в формировании противовирусного иммунного ответа играют натуральные киллеры (NK-клетки), которые уничтожают вирус-инфицированные и опухолевые клетки посредством гранул, содержащих перфорин и гранзимы, внеклеточных лиганд, включая Fas-лиганды (FasL) и лиганд, индуцирующих апоптоз и экзоцитоз цитолитических гранул [1]. Взаимодействие вируса с клеткой хозяина происходит при взаимодействии со специфическими рецепторами на плазматической мембране либо с помощью клатрин- или кавеолин-зависимого эндоцитоза вирусной частицы. Проникновение вируса Эпштейна–Барр (ВЭБ) осуществляется посредством экспрессии рецептора CD21 и ко-рецептора HLA-II после межклеточного взаимодействия [2]. При взаимодействии NK-клеток с CD21+ ВЭБ-инфицированными клетками они могут временно приобретать слабый фенотип CD21+ за счет синаптического переноса небольшого количества рецепторных молекул на собственную мембрану. Эти эктопические рецепторы позволяют ВЭБ связываться с новой NK-клеткой хозяина. Вирусы способны ингибировать цитотоксичность NK-клеток или индуцировать апоптоз, что играет роль в механизме уклонения от иммунного ответа. В эксперименте in vitro установлено, что при инфицировании ВЭБ происходит деформация NK-клеток и увеличение их размера [3]. Ингибирование или изменение небольшой субпопуляции NK-клеток оказывает существенное влияние на формирование адаптивного иммунного ответа на вирусные инфекции [4, 5].
Цитолитическую функцию NK-клеток могут инициировать различные процессы, включая дегрануляцию и лигирование рецепторов смерти, которые являются критическими для скорости очищения как больных, так и дисфункциональных клеток [6]. При стимуляции NK-клетки продуцируют интерферон-гамма (IFN-γ) и фактор некроза опухоли-альфа (TNF- α), они обладают цитолитическими функциями, которые сходны с функциями цитотоксических CD8+-лимфоцитов [7]. Цитотоксичность NK-клеток регулируют 3 основных процесса: распознавание клеток-мишеней; контакт с клетками-мишенями и образование иммунологического синапса (IS); индуцированная NK-клетками гибель клеток-мишеней. Механизм гибели включает активацию рецепторов смерти, присутствующих на поверхности клетки-мишени, в результате инициируется внешний апоптотический путь. Цитотоксичность NK-клеток обусловлена высвобождением литических гранул в клетку-мишень посредством слияния мембран в IS. В результате происходят реорганизация цитоскелета и полимеризация актина в IS, поляризация центра микротрубочек в направлении клетки-мишени [8]. Поляризованные литические гранулы сливаются с мембраной клетки-мишени и высвобождают ферменты, которые активируют в ней программу внутреннего апоптоза.
Компартмент NK-клеток периферической крови человека составляет от 5 до 15% лимфоцитов и состоит из разных стадий дифференцировки. Роль NK-клеток при хронической ВЭБ-инфекции до конца не изучена. При воздействии ВЭБ-инфицированных В-клеток, экспрессирующих литические антигены, часть NK-клеток дегранулирует и пролиферирует. По выраженности экспрессии CD56 компартмент NK-клеток крови состоит из 2 субпопуляций: CD56brightCD16 и CD56dimCD16+ [9]. CD56brightCD16 продуцируют большое количество цитокинов, после длительной активации приобретают цитотоксичность, обогащаются во вторичных лимфоидных органах, где дифференцируются до CD56dimCD16+ NK-клеток, составляя в периферической крови около 90%. CD56dimCD16+ представлены зрелыми терминально дифференцированными NK-клетками с выраженными цитотоксическими свойствами [10].
Из гемолимфы светлячка Calliphoravicina были получены катионные пептиды аллоферон 1 и 2. Они состоят из 12 и 13 аминокислотных остатков (HGVSGHGQHGVHG и GVSGHGQHGVHG соответственно). Аллоферон 2 соответствует усеченной на N-конце форме аллоферона 1. Наиболее активным из них является аллоферон 1, который: 1) стимулирует естественную цитотоксичность лимфоцитов периферической крови человека; 2) индуцирует продукцию IFN в эксперименте у мышей и людей; 3) повышает противовирусную и противоопухолевую резистентность у мышей [11]. В 2003 г. в Российской Федерации разработан препарат аллокин-альфа («Бренд-Фарм», Россия) – антивирусный препарат нового типа, разработанный международным коллективом ученых (регистрационный № 002829/01 от 22.09.2003). Действующим веществом препарата является цитокиноподобный пептид аллоферон (гистидил-глицил-валил-серил-глицил-гистидил-глицил-глутамил-гистидил-глицил-валил-гистидил-глицин).
Цель исследования – изучение влияния аллоферона на содержание количества копий ДНК ВЭБ в образцах слюны методом Real-time ПЦР на динамику содержания натуральных киллеров и изменения цитотоксической активности NK-клеток у больных хронической вирусной инфекцией Эпштейна–Барр (ХВИЭБ) через 6 нед. после окончания терапии.
Материалы и методы
В исследование были включены 100 пациентов, страдающих ХВИЭБ. Группа состояла из 69 женщин и 31 мужчины. Средний возраст пациентов –34,64 ± 1,21 года, длительность течения заболевания – 2,83 ± 0,86 года. У 73 (73%) больных в детстве часто обострялся хронический тонзиллит, не поддающийся терапии антибиотиками, а 25 (25%) больных перенесли острый инфекционный мононуклеоз. Диагноз ХВИЭБ был выставлен и подтвержден лабораторно при обследовании у соответствующих специалистов на предыдущем этапе, и пациенты были направлены на лечение к иммунологу. Больные, которые получали противовирусную или иммуномодулирующую терапию в течение последних 6 мес. не были включены в исследование.
Клиническое исследование выполнено с соблюдением Хельсинкской декларации Всемирной медицинской ассоциации «Этические принципы проведения научных медицинских исследований с участием человека» (WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects, 2013), протокола Конвенции Совета Европы о правах человека и биомедицине 1999 г. и статей 20, 22, 23 Федерального закона «Об основах охраны здоровья граждан в Российской Федерации» от 21.11.2011 № 323-ФЗ (ред. от 26.05.2021). Клиническое исследование проведено по протоколу, одобренному локальным этическим комитетом при ООО «Центр Диализа Санкт-Петербурга» FRESENIUS MEDICAL CARE (Россия). Все участники подписали добровольное информированное согласие. При включении в исследование у пациентов не были выявлены никакие другие инфекции, хронические заболевания или изменения в системе иммунного статуса, которые могли повлиять на результаты проводимого исследования.
Клинические методы исследования включали сбор анамнеза, данные о ранее проводимой терапии, наличии сопутствующих заболеваний. Клиническое состояние пациентов оценивали по общепринятой методике, включающей объективные данные и жалобы пациента на момент осмотра. Жалобы пациента регистрировали с использованием шкалы субъективной оценки по 3-балльной шкале (0 – отсутствие симптомов, 1 – слабая выраженность симптомов, 2 – умеренная выраженность симптомов, 3 – значительная выраженность симптомов). Пациенты были разделены на 2 группы для проведения разных схем терапии. В 1-ю группу вошли 70 чел., которые получали терапию аллофероном (9 инъекций п/к по 1,0 мг через день). Подкожное введение аллоферона хорошо переносилось больными, не вызывало аллергических реакций, не оказывало гепато-нефротоксического и токсического действия на кроветворные органы. Во 2-ю группу (контрольную) были включены 30 больных, которые получали пролонгированную схему терапии валацикловиром (валтрекс) – препаратом из группы ациклических нуклеозидов (500 мг х 2 раза в сутки внутрь) в течение 2 мес.
У пациентов определяли ДНК вируса в образцах слюны методом ПЦР с гибридизационно-флуоресцентной детекцией в режиме реального времени. Использовали тест-системы «АмплиСенс EBV/CMV/HHV6-скрин-FL» (Центральный НИИ эпидемиологии Роспотребнадзора, Россия). Единицы измерения, используемые для оценки вирусной нагрузки при экстракции ДНК из слюны – количество копий ДНК ВЭБ на 1 мл образца (ККДНК). Согласно инструкции, этот показатель рассчитывается по формуле:
ККДНК = КДНК × 100,
где КДНК – количество копий ДНК вируса в пробе.
Аналитическая чувствительность тест-системы составляет 400 копий/мл.
Цитотоксическую активность NK-клеток оценивали по спонтанной и индуцированной экспрессии CD107a – LAMP (Lysosomal-associated membrane protein), экспрессия которого на клеточной мембране лимфоцитов свидетельствует о дегрануляции лизосом. Оценка экспрессии CD107a проводится после совместного культивирования мононуклеаров периферической крови (МНПК) с клетками-мишенями, в качестве которых используется клеточная линия К562 (хронический эритромиелоз человека). Клетки K562 экспрессируют ряд лиганд (MICA, MICB, ULBP2, ULBP4) для рецептора NKG2D цитотоксических лимфоцитов. Взаимодействие NKG2D с лигандами приводит к дегрануляции лизосом NK-клеток, TNK-клеток и лимфокин-активированных CD8+-лимфоцитов и экспрессии на их мембранах CD107a. Таким образом, тест позволяет оценить способность NK-клеток участвовать в NKG2D-зависимом цитолизе клеток-мишеней. Взятие крови на исследование проводится в вакутейнер с гепарином лития в качестве антикоагулянта. Пробоподготовка включает выделение взвеси мононуклеаров периферической крови на градиенте плотности с последующей отмывкой, кокультивирование МНПК и К562 в соотношении 10:1 в условиях CO2-инкубатора в течение 20 ч в присутствии моноклональных антител анти-CD107a-AlexaFluor 647 (BioLegend, США), окрашиванием моноклональными антителами анти-CD3-FITC/CD(CD16+56)PE и анти-CD45PC5 (Beckman Coulter, США). Для оценки спонтанной цитотоксической активности во взвесь МНПК вместо К562 вносили питательную среду RPMI (ООО «БиолоТ», Россия) в соответствующем объеме. Анализ образцов проводился на проточном цитофлюориметре Navios (Beckman Coulter, США). Накопление проводили до 1000 событий в минимальной субпопуляции клеток (NK- или TNK-). Популяцию лимфоцитов определяли как CD45+brightSSdim клетки. Оценивали относительное количество клеток, экспрессирующих CD107a (CD107a+), в субпопуляциях NK-, TNK- и Т-лимфоцитов. Индекс стимуляции рассчитывали как соотношение индуцированной экспрессии CD107a и спонтанной.
Определение относительного количества NK-клеток проводили методом многоцветной проточной цитометрии в рамках исследования субпопуляционного состава лимфоцитов периферической крови. Кровь из локтевой вены брали в вакутейнеры с ЭДТА. Пробоподготовку проводили согласно инструкции производителя. Использовали моноклональные антитела anti-HLADR-FITC, anti-CD4-PE, anti-CD3-ECD, anti-CD56-PC5.5, anti-CD25-PC7, anti-CD8-APC, anti-CD19-APC-AF700, anti-CD45-APC-AF750. Для лизиса эритроцитов применяли VersaLyse. Пробы анализировали на проточном цитофлюориметре Navios (прибор и реактивы производства Beckman Coulter, США). Накапливали 5000 событий в лимфоцитарном регионе CD45+brightSSdim. NK-лимфоциты определяли как CD3-CD56+ CD45+brightSSdim события. Абсолютное количество NK-клеток рассчитывали на основании результатов клинического анализа крови.
Статистическую обработку полученных данных проводили с использованием программного пакета IBM SPSS Statistics, 26 версия (Armonk, NY: IBM Corp.). Групповые результаты представлены в виде средней арифметической ± стандартная ошибка (М ± m). Статистическое сравнение данных между группами больных проводилось с использованием точного критерия Фишера, непараметрического U-критерия Манна–Уитни. Для статистического сравнения данных между группами применяли непараметрический U-критерий Манна–Уитни. Различия между группами считались значимыми при р ≤ 0,05.
Результаты
Перед началом терапии и через 6 нед. после ее окончания у больных в обеих группах определяли количество копий ДНК ВЭБ в образцах слюны, цитотоксическую активность натуральных киллеров, содержание NK-клеток в крови.
В 1-й группе содержание ДНК до начала терапии составляло 321 873,65 ± 46 072,32 копий/мл (Ме – 286 900,00; 95% ДИ – 258 576,36–418 686,02), через 6 нед. после ее окончания – 5847,35 ± 2020,39 (Ме – 4682,40; 95% ДИ – 4503,53–22 430,8; р = 0,0001). В этой группе у 38 (54,28%) больных ДНК ВЭБ не обнаружена. Во 2-й группе (n = 30) содержание ДНК до начала терапии составляло 273 837,25 ± 43 202,14 (Ме – 43 359,88; 95% ДИ – 27 720,27–628 486,02; р = 0,001). В этой группе у 9 (30,0%) больных ДНК ВЭБ не обнаружена. Таким образом, эффективность противовирусной терапии в 1-й группе была значимо выше (р = 0,001); (р = 0,03; тест Фишера).
В табл. 1 представлены показатели содержания NK-клеток до начала и после проведения терапии в обеих группах.
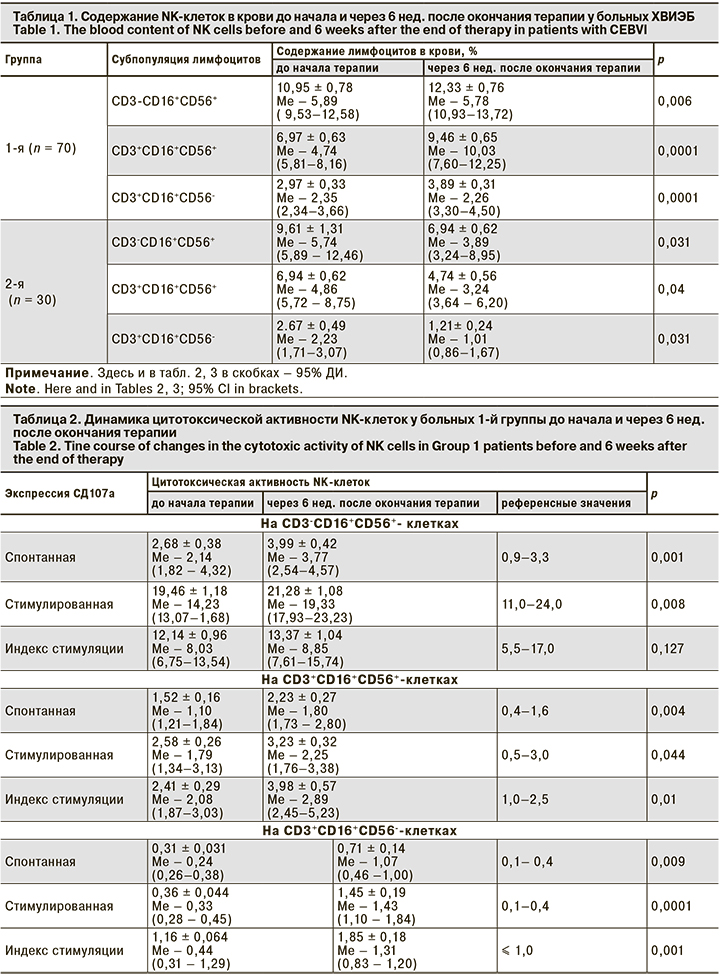
Из представленных данных видно, что содержание NK-клеток крови у больных 1-й группы после терапии аллофероном достоверно увеличилось, у больных в 2-й группы достоверно снизилось. Далее была проведена оценка динамики цитотоксической активности NK-клеток в обеих группах до начала терапии и через 6 нед. после ее окончания (табл. 2, 3).

Из данных табл. 2 видно, что спонтанная и стимулированная экспрессия СD107а на СD3-СD16+СD56+-клетках через 6 нед. после окончания терапии аллофероном достоверно увеличивается, не превышая референсных значений, а на СD3+СD16+CD56+- и СD3+СD16+CD56--клетках достоверно увеличивается, превышая их. То есть терапия аллофероном стимулирует спонтанную и индуцированную дегрануляцию NK-клеток у больных ХВИЭБ.
В отличие от показателей больных 1-й группы во 2-й группе через 6 нед. после окончания терапии валацикловиром спонтанная и стимулированная экспрессия СD107а на СD3-СD16+CD56+-, СD3+СD16+CD56+- и СD3+СD16+CD56--клетках достоверно уменьшается. Таким образом, валацикловир, вероятно, ингибирует спонтанную и индуцированную дегрануляцию NK-клеток у больных ХВИЭБ.
Обсуждение
Элиминация вирус-инфицированных клеток осуществляется посредством цитотоксической активности NK-клеток [12]. Отличительной чертой активации NK-клеток является дегрануляция, то есть высвобождение содержимого литических гранул на поверхности клетки-мишени. Внутренняя поверхность гранул покрыта CD107a (lysosome-associated membrane protein 1) – высокогликозилированным белком, который появляется на поверхности клетки вследствие слияния лизосом с плазматической мембраной. Дегрануляция приводит к экспрессии CD107a на поверхности клеток и истощению внутриклеточного перфорина. После дегрануляции CD107a экспонируется на поверхности цитотоксического лимфоцита, защищая мембрану от перфорин-опосредованного повреждения. Поляризация и дегрануляция цитолитических гранул – это 2 этапа цитотоксичности NK-клеток, которые контролируются отдельными сигналами, исходящими от различных рецепторов. В частности захват интегрина LFA-1 его лигандом ICAM-1 сигнализирует о поляризации, тогда как захват CD16 его лигандом IgG Fc вызывает дегрануляцию. Ни поляризация, ни дегрануляция не являются достаточными для эффективного лизиса клеток-мишеней. Способность NK-клеток к уничтожению вирус-инфицированных клеток происходит до «истощения» NK-клеток, что, вероятно, частично связано с истощением цитолитических гранул [13].
Показано, что результаты анализа дегрануляции NK-клеток коррелируют со стандартными результатами цитотоксичности. То есть экспрессия CD107a может быть чувствительным маркером для определения цитотоксической активности [14]. В нашей работе выявлено достоверное повышение спонтанной и индуцированной экспрессии СD107а на СD3-СD16+CD56+-, СD3+СD16+CD56+- и СD3+СD16+CD56--клетках через 6 нед. после окончания терапии аллофероном. Препарат индуцирует цитотоксическую активность у больных ХВИЭБ. В своей работе Naeun Lee и соавт. [15] показали выраженную противовирусную активность аллоферона, повышающего регуляцию цитотоксичности NK-клеток, что опосредовано усилением секреции перфорина/гранзима. Полученные нами результаты полностью подтверждают ранее опубликованные данные об эффективности аллоферона в стимуляции цитотоксической активности NK-клеток.
В работе D. Thomas и соавт. [16] было продемонстрировано влияние терапии валацикловиром на содержание в крови NK-клеток до и после проведения терапии у женщин, страдающих бесплодием на фоне длительной хронической герпесвирусной инфекции. Авторы показали, что терапия валацикловиром приводит к достоверному снижению содержания NK-клеток в крови у всех пациенток (р = 0,0056), максимальное снижение было выявлено у 73,8% обследованных. В нашем исследовании у пациентов 2-й группы, получавших валацикловир в течение 2 мес. через 6 нед. после окончания терапии было выявлено достоверное снижение содержания NK-клеток в периферической крови и экспрессии СD107а на их мембране. Это полностью соответствует результатам влияния валацикловира на уровень NK-клеток, полученным ранее другими исследователями.
В 1-й группе больных, получавших аллоферон, через 6 нед. после терапии отмечалось достоверное увеличение содержания NK-клеток, что свидетельствует о влиянии препарата на их экспансию. Известно, что соединение [3-13]-аллоферон(1) проявляет наиболее сильную противовирусную активность, ингибирует репликацию герпесвирусов и индуцирует продукцию IFN у людей [17]. С.И. Черныш и соавт. [18, 19] показали, что через 2 ч после введения аллоферона содержание IFN становится в 2–2,5 раза выше обычного фонового, сохраняясь на повышенном уровне в течение 6–8 ч и снижаясь до исходных значений к концу суток. Авторы делают вывод о том, что препарат действует как индуктор IFN. На основании этих данных можно предположить, что механизм экспансии СD3-СD16+CD56+-клеток и увеличения спонтанной экспрессии СD107а на СD3-СD16+CD56--клетках является сочетанным, то есть за счет как действия самого аллоферона, так и периодического кратковременного повышения продукции IFN-α в процессе курса терапии этим препаратом.
Выводы
1. Аллоферон оказывает выраженный эффект на снижение количества копий ДНК ВЭБ в образцах слюны у больных ХВИЭБ через 6 нед. после окончания терапии.
2. Через 6 нед. после терапии аллофероном отмечалось достоверное увеличение содержания NK-клеток, что свидетельствует о влиянии препарата на их экспансию у больных ХВИЭБ.
3. Терапия аллофероном стимулирует спонтанную и индуцированную дегрануляцию NK-клеток, то есть цитотоксическую активность у больных ХВИЭБ.


