Хроническая HBV-инфекция является динамическим процессом, отражающим взаимодействия между вирусом гепатита В (HBV) и иммунным ответом хозяина, в связи с чем не у всех пациентов развивается хронический гепатит В (ХГВ). Эпидемиология острого гепатита В (ОГВ) меняется в результате воздействия нескольких факторов, в том числе миграции населения и политики вакцинации. Несмотря на накопленный опыт в изучении HBV-инфекции, все еще остается много невыясненных вопросов, которые требуют дальнейших исследований.
По оценкам ВОЗ, по состоянию на 2019 г. примерно 296 млн чел. в мире хронически инфицированы HBV, экспрессируя ДНК HBV и поверхностный антиген ГВ (HBsAg) в крови. Проведенные исследования продемонстрировали, что в 2019 г. из 296 млн инфицированных о своем диагнозе знали менее 10%, а лечение получали менее 2%. При этом по данным ВОЗ, на 2021 г. в немедленной терапии нуждались от 12 до 25% больных ХГВ [1] (рис. 1).
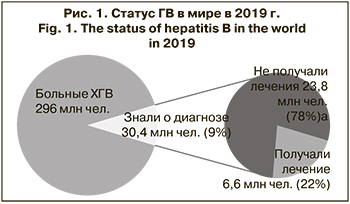
Так как многим пациентам диагноз ставят уже на поздних стадиях заболевания печени, смертность от последствий HBV-инфекции, включая цирроз печени (ЦП) и гепатоцеллюлярную карциному (ГЦК) [2], постоянно растет: число смертей от заболеваний печени, связанных с ГВ, увеличилось с 786 000 в 2010 г. до 820 000 в 2019 г. [1, 3]. Это происходит несмотря на вакцинацию и разработку ряда препаратов с противовирусным потенциалом для лечения пациентов в ХГВ. Кроме того, бремя, которое несет мировое здравоохранение от ГВ и его последствий, значительно тяжелее, чем считали ранее, так как практически нет учета скрытых форм и случаев реактивации ГВ.
Для снижения заболеваемости и смертности от ХГ необходимо продолжать усилия по выявлению инфицированных лиц путем целенаправленного скрининга, профилактики новых инфекций с помощью вакцинации, а также мониторинга и лечения лиц, подверженных риску осложнений ХГВ, включая эпиднадзор за ГКЦ [4–6].
В 2017 г. Европейская ассоциация по изучению печени (EASL) представила обновленные рекомендации, в которых предлагается классифицировать хроническую HBV-инфекцию на 5 фаз с учетом наличия HBeAg, уровней HBV ДНК, значения аланинаминотрансферазы (AЛT) и наличия воспаления печени: [7]: I – HBeAg-позитивная хроническая инфекция; II – НВeАg-позитивный хронический гепатит; III – HBeAg-негативная хроническая инфекция; IV – HBeAg-негативный хронический гепатит и V – HBsAg-негативная фаза или скрытый ГВ. Таким образом, новая номенклатура основана на описании двух основных характеристик хронизации: инфекция/гепатит. Люди с хронической HBV-инфекцией могут переходить из одной иммунологической фазы в другую, и причины этого перехода не известны [8, 9].
В настоящее время EASL рекомендует 2 стратегии терапии хронической HBV-инфекции, направленные на исчезновение HBsAg или, по меньшей мере, невозможность обнаружения ДНК HBV методом ПЦР [7].
Первая стратегия – лечение с помощью Пег-ИФН в течение ограниченного периода времени (обычно 48 нед.), вторая – лечение на неопределенный период времени аналогами нуклеозидов (НА), которые подавляют репликацию вируса путем ингибирования обратной транскриптазы [7].
В рекомендациях подчеркивается, что среди пероральных противовирусных средств предпочтение следует отдавать энтекавиру, тенофовиру дизопроксил фумарату и тенофовиру алафенамиду как препаратам с выраженной противовирусной активностью и высоким генетическим барьером к развитию резистентности [9, 10].
Американская ассоциация по изучению заболеваний печени (AASLD) не рекомендует начинать лечение, если уровень АЛТ менее чем в 2 раза выше нормы при условии отсутствия воспаления или фиброза печени, подтвержденного при биопсии [11]. Препараты первой линии для людей с концентрациями АЛТ, более чем в 2 раза превышающими нормальные уровни, совпадают с препаратами, рекомендованными EASL. Длительность терапии НА должна составлять, как минимум, 1 год, а продолжить лечение после HBeAg-сероконверсии у HBeAg-позитивных пациентов предпочтительно на 12 мес. При этом частота сероконверсии в течение 5 лет терапии превышает 50%. Репликация HBV подавляется более чем у 95% пациентов.
При HBeAg-негативном ХГВ длительность лечения энтекавиром или тенофовиром не определена, предполагается, что она должна быть длительной (более 5 лет) и проводиться до наступления сероконверсии HBsAg, а это за указанный срок происходит не более чем в 10% случаев. Репликация HBV на фоне терапии контролируется у более 95% пациентов при приеме энтекавира и тенофовира в течение 3–5 лет и более при соблюдении рекомендованного режима приема препаратов В результате столь длительного лечения отмечен значительный регресс фиброзных и цирротических изменений в печени [12, 13]. Однако при отмене НА возможна реинфекция ХГВ, поэтому непонятно, как долго следует продолжать противовирусную терапию (ПВТ) и существуют ли маркеры отмены лечения.
При этом известно, что скорость серологического выздоровления (функционального излечения – потеря HBsAg с сероконверсией в anti-HBs) при приеме пероральных противовирусных препаратов не намного выше, чем спонтанная скорость сероконверсии HBsAg – около 1% в год, поэтому лечение часто длится всю жизнь [14, 15]. Истинное же излечение, при котором удаляются и HBsAg, и сссDNA, является целью будущей ПВТ.
Таким образом, несмотря на многолетнее изучение HBV-инфекции, лечение больных по-прежнему остается сложной клинической задачей и в большинстве случаев оказывается недостаточно эффективным.
Цель исследования – определение особенностей течения ХГВ на фоне ПВТ.
Материалы и методы
Обследовано 70 HBsAg-позитивных пациентов, из них 24 (34,3%) получали терапию НА и 46 (65,7%) наблюдались без лечения. Клинические методы включали сбор анамнеза, объективное обследование, биохимический анализ крови (билирубин общий/прямой, АЛТ, АСТ, ГГТ, ЩФ), выявление специфических маркеров гепатитов В, С, D методом ИФА, количественный анализ ДНК HBV методом ПЦР (чувствительность – 10 МЕ/мл). Всем больным ХГВ в динамике была проведена фиброэластометрия на аппарате «Фиброскан».
Статистический анализ результатов проводили с использованием программ IBM SPSS Statistics 24 (IBM) и Microsoft Office Excel 2016 (Microsoft). Определяли процентное выражение ряда данных, среднюю арифметическую (М) и стандартную ошибку средней арифметической (m). Для сравнения количественных значений в зависимости от характера распределения значений выборки использовали t-критерий Стьюдента и U-критерий Манна–Уитни.
Результаты
Длительность наблюдения составляла от 1 года до 15 лет. 58% пациентов были в возрасте до 40 лет, число мужчин и женщин было равным. Пациенты с сопутствующими заболеваниями в целом составляли 46%, но в группе больных ХГВ, получающих ПВТ, сопутствующие заболевания выявляли гораздо чаще, чем в группе больных без лечения – у 71 и 30% соответственно (р < 0,05).
60 (86%) пациентов были HBe-негативными. Причем активность воспалительного процесса у пациентов с е-антигеном и без него была обратно пропорциональной: в группе HBeAg-позитивных пациентов явления гепатита регистрировали в 70% случаев, в то время как у HBeAg-негативных – менее чем в 30% (рис. 2).

Большинство HBsAg-позитивных пациентов не получали и в настоящее время не получают ПВТ. 34% пациентов получали НА, из них 58% были наивными, а 42% имели в анамнезе прерванный курс ПВТ интерферонами или НА (рис. 3).
У 40 (57%) пациентов длительность обнаружения HBsAg на момент первичного обращения составляла менее 5 лет. Число пациентов с длительностью выделения HBsAg более 20 лет совпадало с числом пациентов с выраженным фиброзом (FЗ–F4) – 8 чел. (11%) (рис. 4).

У 58 (83%) пациентов при первичном обращении фиброз отсутствовал или определялся в минимальной степени. Цитолитический синдром (более 3 норм), который выявляли при первичном обращении в 42% случаев, после назначения ПВТ купировался. В 21% случаев небольшой цитолиз (до 3 норм) сохранялся. На фоне ПВТ число пациентов с нормальными показателями трансаминаз увеличилось с 9 (37,5%) до 19 (79,2%) чел. ( р < 0,05).
Если при первичном обращении минимальная вирусная нагрузка (ВН) определялась у 13 (54,2%) пациентов, умеренная – у 4 (16,7%) и высокая – у 7 (29,2%), то на фоне ПВТ удельный вес больных с минимальной ВН сократился почти в 5 раз – с 54,2 до 12,5% ( р < 0,05). ДНК HBV перестала определяться ультрачувствительным методом ПЦР почти в 30% случаев (у 7 чел.). В 58,3% случаев (у 14 чел. из 24) ДНК выявлялась, однако ее количество определить было невозможно, так как оно было ниже минимальных референсных значений.
При анализе стадий фиброза печени через 5–7 лет установлено, что в группе больных, не получавших ПВТ, стадию фиброза F3–F4 регистрировали в 17,4% случаев (8 чел. из 46) против 8,3% (2 чел. из 24) на фоне ПВТ (р < 0,05). Приверженность терапии составила 41,7% (10 чел. из 24).
Обсуждение
Пероральная терапия НА улучшает и восстанавливает функцию даже у пациентов с тяжелым декомпенсированным заболеванием печени [15]. Устойчивое подавление вируса предотвращает прогрессирование фиброза и приводит к его регрессу даже у пациентов с установленным ЦП. В регистрационном исследовании тенофовира через 5 лет наблюдения результаты парной биопсии печени показали, что из 348 пациентов у 87% был выявлен регресс фиброза, в том числе у 71 (74%) из 96 пациентов с ЦП [16]. Результаты нашего исследования совпадают с вышеуказанными данными: из 8 чел. с фиброзом F3–F4 через 5–7 лет в группе пациентов, получавших ПВТ НА, осталось только 2, а у 6 (75%) пациентов произошел регресс фиброза. Устойчивое подавление вируса также снижает риск гепатоканцерогенеза [17]. Ни у одного из наших пациентов, получающих ПВТ, не был установлен диагноз ГЦК.
Заключение
У 86% пациентов была выявлена HBeAg-негативная HBV-инфекция и начальная степень фиброза (у 83%). ПВТ назначали независимо от стадии фиброза, чаще пациентам в возрасте 41–60 лет (в 41,7% случаев) с ВН от 5 lоg. При этом длительность обнаружения HBsAg на момент начала терапии составила менее 5 лет у 58,3% больных. Из пациентов, начавших принимать НА, меньше половины остались привержены терапии. При этом стадия фиброза печени у тех, кто получал ПВТ, уменьшилась, что, возможно, свидетельствует об антифибротическом эффекте НА.
Таким образом, на сегодняшний день отсутствуют четкие стандарты ведения пациентов с хронической HBV-инфекцией. Современные противовирусные методы лечения ХГВ подавляют репликацию вируса, но малоэффективны для элиминации самого вируса, в связи с чем большинство пациентов с хронической HBV-инфекцией вынуждены принимать НА неопределенно длительный срок, что полностью соответствует сегодняшней стратегии достижения функционального излечения. Как показывает наше собственный опыт, это оправдано в период отсутствия новых терапевтических подходов к лечению пациентов с ХГВ, так как устойчивое подавление вируса предотвращает прогрессирование фиброза и приводит к его регрессу даже у пациентов с установленным ЦП.


