ВИЧ-инфекция приводит к прогрессирующей потере количества и изменению функций CD4+-лимфоцитов, нарушению иммунного ответа и формированию вторичных оппортунистических инфекций [1–2]. Орофарингеальные оппортунистические инфекции встречаются у большинства ВИЧ-инфицированных пациентов, часто вызывая выраженные поражения, которые способствуют ухудшению питания с последующей потерей массы тела. Эпителий полости рта при ВИЧ-инфекции становится более проницаемым. Исследования на модели приматов показали, что в слизистой оболочке кишечника [3, 4] и полости рта инфекция вирусом иммунодефицита обезьян (SIV) приводит к быстрому изменению генов, обеспечивающих регенерацию эпителия [5]. В дополнение к увеличению проницаемости барьера нарушение регенеративной способности эпителия усиливает восприимчивость к ОИ, способствует нарушению гомеостаза микробиоты. LPS-связывающий белок (LBP) играет центральную роль в ответе на эндотоксинемию и достаточно информативно отражает активность инфекционного процесса при ВИЧ-инфекции.
Микробиом человека представляет собой сложное полимикробное сообщество с тонким балансом [6]. Микробиота состоит из множества аэробных и анаэробных видов, которые продуцируют среду пептидов и полисахаридов, взаимодействуют друг с другом для поддержания стабильной симбиотической микросреды. В здоровых тканях полости рта доступ к эпителию надежно защищен от некомменсальных организмов, частично благодаря физическим и физиологическим барьерам, создаваемым микробиомом. Микробные антигены, такие как липополисахарид, флагеллин, пептидогликан, способствуют этому процессу. Эти антигены по-разному стимулируют механизмы врожденного ответа через рецепторы распознавания антигенов (PRR) и тем самым регулируют местную физиологическую среду [7].
Исследования показали, что состав микробиома ротоглотки изменяется вследствие ВИЧ-инфекции даже на фоне антиретровирусной терапии (АРТ) [8–14]. При ВИЧ-инфекции отмечается повышение количества грибов рода Candida [10, 12, 13], а также увеличение концентрации ДНК ВПЧ, несмотря на проводимую АРТ [14].
Орофарингеальные заболевания, часто наблюдаемые у больных ВИЧ-инфекцией, способствуют кардиометаболическим нарушениям [15]. Предполагается, что избыточный рост патогенных бактерий (например, Fusobacteria, Prevotella, Porphyromonas spp.) вызывает эндотоксемию с последующим системным воспалением [16]. Повторное воздействие эндотоксина приводит к увеличению риска развития фиброза миокарда и смертности [17].
Полученные данные предполагают связь между бактериальными сообществами полости рта и ротоглотки с системными заболеваниями, однако окончательную роль еще предстоит определить, особенно в отношении больных ВИЧ-инфекцией, поскольку проведенные ранее исследования ограничены небольшими размерами выборки и вариациями в методологии анализа. Это подчеркивает необходимость продолжения изучения характеристики микробиома ротоглотки у больных ВИЧ-инфекцией, получающих АРТ, а также поиска новых терапевтических подходов в дополнение к АРТ.
С целью коррекции нарушений микробиоты ротоглотки представляется перспективным изучение эффективности применения препаратов с противовоспалительным и иммуномодулирующим действием. Основные фармакологические эффекты аминодигидрофталазиндиона натрия (АДФNa, галавит®) обусловлены его влиянием на репаративные процессы в тканях, а также снижением образования фиброзной ткани при заживлении, повышением неспецифической резистентности организма к инфекционным заболеваниям [18]. В клинических исследованиях было показано положительное влияние АДФNа на нормализацию показателей иммунного статуса и цитокинового профиля при целом ряде неинфекционных и инфекционных заболеваний, в том числе вирусной этиологии [19–21].
Учитывая вышеизложенное, целью настоящей работы явилось изучение особенностей состава микробиоты ротоглотки и системного воспаления у больных ВИЧ-инфекцией на фоне АРТ и оценка возможности их коррекции АДФNа.
Материалы и методы
В исследовании приняли участие 100 больных ВИЧ-инфекцией в возрасте 39,4 ± 2,2 года. Все пациенты в течение не менее 1 года получали АРТ, назначенную в соответствии с современными нормативными документами, действующими на момент проведения исследования1. Рандомизацию проводили методом случайной выборки. Пациенты были распределены на 2 равные группы по 50 человек в каждой. По основным клиническим параметрам, возрасту и полу, стадии ВИЧ-инфекции, уровню CD4+-лимфоцитов группы были сопоставимы, что и позволило провести в дальнейшем их сравнение (табл. 1). В соответствии с клиническим протоколом исследования клинико-лабораторное обследование пациентов проводили исходно (1-й визит) и через 4 нед. (2-й визит) при плановом диспансерном наблюдении.
Пациентам 1-й группы при 1-м визите помимо планового наблюдения и лечения дополнительно к стандартной АРТ назначали сублингвальные таблетки АДФNa: по 2 таблетки 2 раза в день в течение 10 дней, затем по 2 таблетки 2 раза через сутки в течение 10 дней. Эффективность терапии оценивали при 2-м визите через 4 нед. наблюдения по результатам динамики концентрации LBP и изменений микробиома ротоглотки.
Исследование концентрации LBP проводили методом ИФА с использованием тест-системы Hbt Human LBP ELISA Kit, Product Number: HK315 (Hycult biotechnology, Голландия).
Показатели, характеризующие состав и наличие изменений микрофлоры слизистой оболочки задней стенки глотки, определяли на бактериологическом анализаторе VITEK-2 соmpact (BioMerieux, Франция) и дискодиффузионным методом. Поверхность задней стенки ротоглотки была выбрана для забора проб микробиома в связи с тем, что на этом анатомическом участке обычно меньше различий в структуре микробного сообщества по сравнению с другими нишами полости рта, а также эта локализация чаще связана с проявлениями ВИЧ-ассоциированных заболеваний полости рта. Использовали стандартную методику забора мазков с дальнейшим проведением качественно-количественного анализа состава выделенных микроорганизмов. Во время забора материала со слизистой оболочки ротоглотки ни у одного пациента не было клинических проявлений заболеваний полости рта и ротоглотки. Кроме того, ни один из участников исследования не получал одновременного симптоматического или профилактического лечения, в том числе антибактериальными или противогрибковыми препаратами. Для выделения микроорганизмов применяли дифференциально-диагностические среды: кровяной агар, среды Сабуро, Эндо, желточно-солевой агар (ФБУН ГНЦ ПМБ, Оболенск, Россия), UriSelect-4 (Bio-Rad Laboratories Inc., США). Идентификацию условно-патогенных микроорганизмов (УПМ) проводили на базе MS Microflex Biotyper MALDI-ToF (Bruker, Германия).
Группа сравнения была сформирована из 30 практически здоровых лиц сопоставимого возраста и пола.
Для статистической обработки использовали программу SPSS Statistics Base 22.0. Проводили определение средних и медианных величин, стандартного отклонения, ошибки средней, достоверности различий, наличия и силы корреляции признаков. Количественные величины представляли как среднее значение (М) ± ошибка средней (m). Определение достоверности различий при сравнении двух групп из совокупностей с нормальным распределением проводили с помощью t-критерия Стьюдента для двух выборок. Критическую величину уровня значимости p принимали равной 0,05, что соответствует критериям, принятым в медико-биологических исследованиях.
Результаты
При рассмотрении качественного и количественного состава микрофлоры общей когорты пациентов в нашем исследовании у 32,0% выявлены представители УПМ в количестве, не превышающем контрольных значений для здоровых лиц. Доминирующими микроорганизмами в биотопе были коагулазоотрицательные стафилококки (Staphylococcus epidermidis и др.) – 22,5%, коринеформные бактерии (Corynebacterium) – 21,5%, альфа-гемолитические стрептококки – 19,5% и Neisseria flavescens – 6,7%.
У остальных 68,0% больных выявлены нарушения микробного состава слизистой оболочки ротоглотки. Всего выделено 59 культур. Чаще всего (в 51,7% случаев) выявляли ассоциации бактерий: двух- (33,3%), трех- (13,3%), четырех- и более компонентные (5,1%).
Среди УПМ с избыточным ростом преобладали стрептококки альфа-гемолитические – 48,0%, коагулазоотрицательные – 32,4%, коринеформные бактерии (Corynebacterium) – 24,5%, Staphylococcus aureus – 22,5% и пневмококки (S. pneumoniae) – 17,6%. С большой частотой (13,7%) выявляли микроорганизмы рода Streptococcus группы А, обладающие бета-гемолитической активностью (S. pyogenes, S. agalactiae и др.). Альфа-гемолитические стрептококки были представлены S. salivarius, S. mitis, S. viridans, S. mutans, S. ovaries. У 17,6% больных был выделен Streptococcus pneumoniae, у 4,0% – негемолитические стрептококки. В единичных случаях выделяли Streptococcus anginosus, S. оralis и S. peroris.
Род Staphylococcus в большинстве случаев был представлен Staphylococcus aureus, а также коагулазоотрицательными стафилококками. Выявлено большое разнообразие коагулазонегативных стафилококков: S. epidermidis, S. haemolyticus, S. saprophyticus, S. cohnii, S. succinus.
Избыточный рост Neisseria отмечен у 16,7% пациентов. В большинстве случаев были выделены Neisseria flavescens, реже – Neisseria perflava, Neisseria subflava, Neisseria mucosa.
Частота выявления микроорганизмов семейства Enterobacteriaceae в биотопе ротоглотки составила 10,8%. Это семейство было представлено преимущественно бактериями рода Escherichia, реже (в 1% случаев) –энтерококками и Klebsiella.
В 10,8% случаев определяли микроорганизмы из группы неферментирующих бактерий: Acinetobacter, Pseudomonas aeruginosa и Chryseobacterium spp. P. aeruginosa, обладающая высокой резистентностью к действию антибактериальных препаратов, была выделена только у 1% больных. Также редко выделяли Chryseobacterium spp. (у 1% больных). При изучении чувствительности к антибактериальным препаратам этих бактерий в 50% случаев выявлена устойчивость ко всем антибиотикам и бактериофагам.
Благодаря применению современного метода масс-спектрометрии при проведении идентификации УПМ, у больных ВИЧ-инфекцией были выделены редко встречающиеся микроорганизмы: Gemella haemolysans, Massilia timonae, Ervinia spp., Pannonibacter phragmitetus и Weissella minor.
Грибковая микрофлора выявлена у 19,6% больных и представлена дрожжеподобными грибами рода Candida. У подавляющего большинства пациентов была выделена Candida albicans. Также среди представителей грибковой микрофлоры в единичных случаях были обнаружены Candida cruzei. У 1 пациентки был обнаружен род дрожжей Pichia семейства Saccharomycetaceae.
При сравнении видового и количественного состава основных представителей резидентной аэробной и факультативной аэробной групп микроорганизмов, выделенных из ротоглотки перед началом исследования в обеих группах, достоверных различий не обнаружено (табл. 2).
При исследовании через 4 нед. в 1-й группе отмечено снижение доли пациентов с избыточным ростом УПМ с 74,0 ± 6,2 до 49,9 ± 7,1% (р < 0,05). Во 2-й группе в этот же период достоверных отличий не зарегистрировано.
Через 4 нед. наблюдения у пациентов 1-й группы снизилась частота регистрации избыточного роста бета-гемолитических стрептококков (S. pyogenes, S. agalactiae и др.) с 20,0 ± 5,7% при первом визите до 6,0 ± 3,4% при втором (р < 0,05). При повторном визите было зарегистрировано также снижение частоты выявления Staphylococcus aureus и дрожжеподобных грибов рода Candida в количестве более 103 КОЕ/мл (табл. 2).
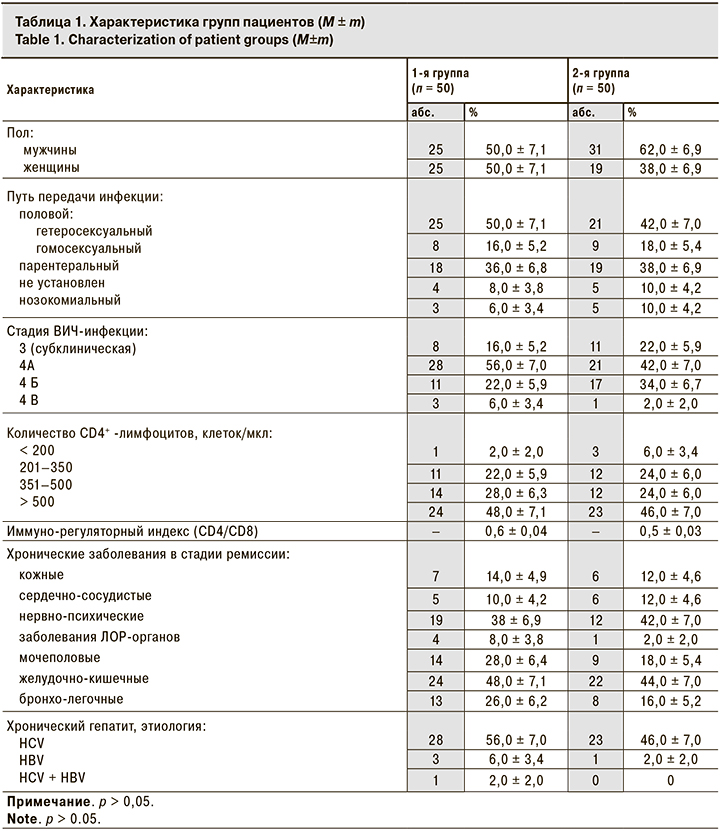
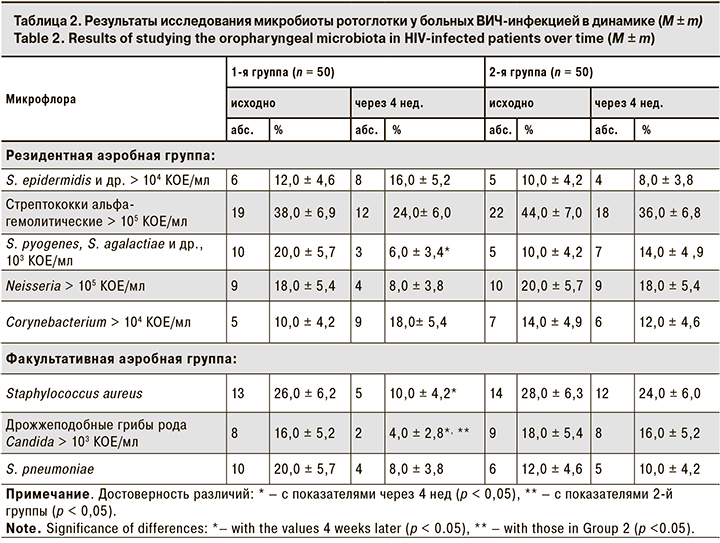
Примечательно, что в 1-й группе снизилась и частота выявления энтеробактерий с 12,0 ± 4,6% на старте лечения АДФNa до 2,0 ± 2,0% через 4 нед. наблюдения, а также ацинетобактерий с 8,0 ± 3,8% до 0 соответственно (рис. 1, 2). При этом во 2-й группе между данными о частоте выявления энтеробактерий и Acinetobacter, полученными при 1-м и 2-м визитах, достоверных отличий не выявлено.
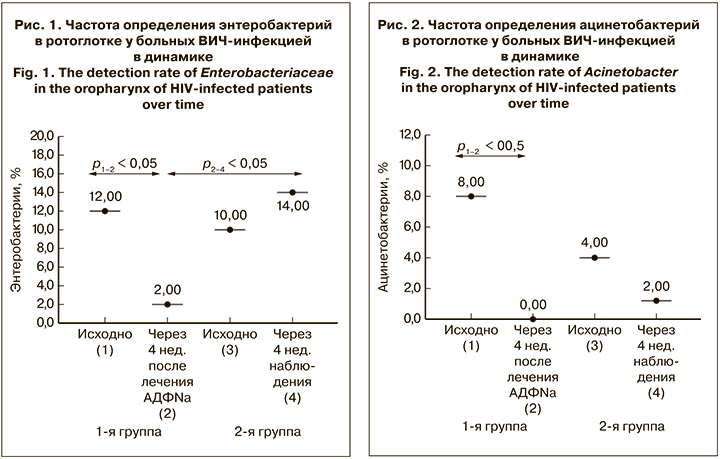
 Концентрация LBP до начала лечения в 1-й и 2-й группах составляла 85,9 ± 3,8 и 86,3 ± 4,8 мкг/ мл соответственно при референсных значениях у здоровых лиц 6,2 ± 1,53 мкг/л (p < 0,01) (рис. 3). При исследовании через 4 нед. в 1-й группе отмечено снижение LBP до 70,9 ± 4,6 мкг/мл (р < 0,05), во 2-й группе достоверных изменений содержания LBP в динамике заболевания не выявлено.
Концентрация LBP до начала лечения в 1-й и 2-й группах составляла 85,9 ± 3,8 и 86,3 ± 4,8 мкг/ мл соответственно при референсных значениях у здоровых лиц 6,2 ± 1,53 мкг/л (p < 0,01) (рис. 3). При исследовании через 4 нед. в 1-й группе отмечено снижение LBP до 70,9 ± 4,6 мкг/мл (р < 0,05), во 2-й группе достоверных изменений содержания LBP в динамике заболевания не выявлено.
Обсуждение
Изучение микробиома человека продолжает оставаться актуальным вопросом, однако сообщения об изменениях в микробиоте у больных ВИЧ-инфекцией все еще ограничены. Последние достижения, обусловленные внедрением современных методов исследования в микробиологии, включая масс-спектрометрию, значительно увеличили наши диагностические возможности для анализа микробных ассоциаций, включая их функциональные изменения, влияющие на течение заболеваний.
Большинство предыдущих исследований перорального микробиома были спланированы как перекрестные, в которых сравнивали показатели ВИЧ-инфицированных участников с ВИЧ-отрицательным контролем [10–12]. В других работах оценивали продольные изменения орофарингеальной микрофлоры после начала АРТ, которые показывают, что ВИЧ и АРТ приводят к изменениям в специфических бактериальных таксонах в микробиоме ротоглотки. Экспрессия этих бактериальных сообществ может также привести к дальнейшей иммунной активации, поскольку микробная транслокация из полости рта может способствовать формированию системного воспаления, подобно эндотоксинемии, развивающейся при попадании в системный кровоток микробных компонентов из просвета кишечника [22].
Современные литературные данные свидетельствуют о возможности использовать определение концентрации LBP в качестве диагностического биомаркера эндотоксинемии и индикатора системного ответа на LPS при различных инфекционных процессах.
Восстановление микроэкологии ротоглотки имеет важное клиническое значение в предотвращении дальнейшего распространения множества ассоциированных между собой микроорганизмов, предупреждая генерализацию и хронизацию патологических очагов инфекции. В нашем исследовании в 10,8% случаев выявлены представители группы неферментирующих бактерий (Acinetobacter, Pseudomonas aeruginosa и Chryseobacterium spp.), играющие основную роль в развитии внутрибольничных инфекций, особенно у иммунокомпрометированных лиц. Каждый выделенный вид микроорганизмов из группы неферментирующих бактерий способен вызывать рецидивирующие инфекции нижних дыхательных путей у больных с выраженным иммунодефицитным состоянием, особенно при многократных курсах антибиотикотерапии [23]. С такой же частотой (10,8%) у пациентов в ротоглотке присутствовали представители семейства Enterobacteriaceae. Как известно, распространение E. coli может вызвать заболевания желудочно-кишечного тракта, геморрагический колит, гемолитико-уремический синдром, инфекции мочевыводящих и желчевыводящих путей, сепсис [23].
Следует обратить внимание и на выделение в 1% случаев редкого возбудителя оппортунистических микозов – дрожжей рода Pichia. В настоящее время они встречаются у пациентов с ослабленным иммунитетом. Известны случаи интерстициальной пневмонии, инфекционного эндокардита, инфекций мочевыводящих путей и слизистой оболочки полости рта, ассоциированные с Pichia [11].
Таким образом, в большинстве случаев микрофлора ротоглотки больных ВИЧ-инфекцией даже на фоне АРТ имеет существенные различия с составом микробиоты аналогичной локализации у здоровых лиц. Потенциально опасным является наличие представителей оппортунистических инфекций, практически не встречающихся у здоровых лиц. С таким составом микробиоты ротоглотки вполне согласуется и высокий уровень LBP, основной функцией которого является связывание эндотоксинов бактерий.
Проведенное исследование продемонстрировало положительное влияние АДФNa на течение ВИЧ-инфекции при добавлении его к стандартной схеме АРТ. Это выразилось в более быстром снижении уровня маркера антиэндотоксиновой защиты LBP, а также числа потенциальных патогенов в микробиоте ротоглотки – бета-гемолитических стрептококков, Staphylococcus aureus, дрожжеподобных грибов рода Candida, энтеробактерий и ацинетобактерий. Вероятно, способность этого препарата оказывать непосредственное или опосредованное влияние на системный воспалительный ответ при ВИЧ-инфекции, в условиях подавления репликации ВИЧ с помощью АРТ, обусловила его позитивное влияние на течение ВИЧ-инфекции. Для определения продолжительности выявленных эффектов, целесообразности и кратности повторных курсов лечения иммуномодулирующими препаратами на фоне АРТ требуются дальнейшие исследования.
Заключение
У подавляющего большинства больных ВИЧ-инфекцией отмечается активация системы антиэндотоксиновой защиты. Анализ видового состава микробиоты ротоглотки показал, что у большей части таких больных отмечается избыточный рост УПМ, в том числе Staphylococcus aureus и дрожжеподобных грибов рода Candida. Доминирующими микроорганизмами в биотопе являются стрептококки. Чаще всего обнаруживаются многокомпонентные ассоциации УПМ с избыточным ростом.
Снижение концентрации LBP и нормализация состава микробиоты ротоглотки у больных ВИЧ-инфекцией наблюдается при дополнении к стандартной АРТ АДФNa. Патогенетическим обоснованием этого является противовоспалительная и иммуномодулирующая активность препарата, связанная с его способностью регулировать функционально-метаболическую активность клеток врожденного и адаптивного иммунитета (в том числе моноцитов, макрофагов, нейтрофилов, натуральных киллеров) и цитокиновый профиль в условиях подавления репликации ВИЧ на фоне АРТ. Курсовой прием АДФNa способствует повышению эффективности и переносимости АРТ.


