Герпесвирусы способны вызывать скрытые пожизненные инфекции. Механизм взаимодействия вирус–вирус при вирусной реактивации герпесвирусов или между ними представляет большой интерес. Вирус герпеса 6-го типа (ВГЧ-6) и вирус Эпштейна–Барр (ВЭБ) часто обнаруживаются одновременно. В L. Cuomo и соавт. показали, что ВГЧ-6, являясь преимущественно Т-лимфотропным вирусом, инфицирует некоторые ВЭБ-позитивные линии В-клеток и вызывает в них активацию литического цикла ВЭБ. Авторы продемонстрировали, что: к инфицированию способен вариант А ВГЧ-6, а инфицирование вариантом В ВГЧ-6 не индуцирует экспрессию антигенов ВЭБ; методом иммуноэлектронной микроскопии выявлено, что присутствие генома ВЭБ способствует восприимчивости линий B-клеток к инфекции ВГЧ-6, увеличивая участки связывания и процент инфицированных клеток; инфицированные ВГЧ-6 Т-клетки демонстрируют сильную трансактивацию промоторов ранних генов ВЭБ BZLF1 и BMRF1 [1]. ВГЧ-6 в 10 раз увеличивает экспрессию раннего гена Zebra, диффузных и ограниченных (85 кДа) ранних генов (EA-D и EA-R соответственно) в обеих ВЭБ-продуцирующих и непродуцирующих клеточных линиях (P3HR1, Akata и Raji). Экспрессия непосредственно раннего гена ВЭБ Zebra приводит к последующей репликации вируса. ВГЧ-6 активирует транскрипцию гена Zebra через циклический AMP-чувствительный элемент (CRE), расположенный в промоторе Zebra (Zp), а область ZII домена Zp является мишенью для трансактивации ВГЧ-6 [2]. Максимальная индукция раннего гена EA-D наблюдается через 72 ч после инфицирования ВГЧ-6. Также после инфицирования ВГЧ-6 в клетках-продуцентах ВЭБ (P3HR1 и Akata) увеличивается экспрессия поздних продуктов гена ВЭБ, а именно вирусного капсидного антигена (125 кДа) и гликопротеина вирусной мембраны gp350. Было обнаружено, что большинство клеток, экспрессирующих гликопротеин вирусной мембраны gp350, также являются положительными для антигенов ВГЧ-6. Это свидетельствует о прямом влиянии репликации ВГЧ-6 на индукцию репликативного цикла ВЭБ. После инфицирования клеток Raji ВГЧ-6 не было выявлено экспрессии поздних генов ВЭБ, что свидетельствует об отсутствии функциональной комплементации между удаленной формой ВЭБ и суперинфекцией ВГЧ-6. Ультрафиолетовое облучение или инактивация высокой температурой ВГЧ-6 приводят к тому, что вирус не активирует экспрессию гена Zebra или EA-D в клетках Raji. L. Flamand и соавт. [3], установили, что ВГЧ-6, являясь лимфотропным герпесвирусом человека, может активировать репликацию ВЭБ и таким образом способствует развитию патогенеза заболеваний, связанных с ВЭБ. После инфекционного мононуклеоза может развиваться синдром постинфекционной хронической усталости, который встречается более чем в 60% случаев, сопровождающихся постоянной активацией инфекции ВГЧ-6. При проведении серологического скрининга 215 случаев острого инфекционного мононуклеоза была выявлена инфекция ВГЧ-6: у 15,3% больных – первичная, у 29,3% – активная инфекция или реактивация ВГЧ-6 и у 33,9% – латентная. Двойная активная инфекция ВЭБ и ВГЧ-6, включая первичные и реактивированные формы, встречалась в 39,5% случаев [4]. Примерно в 1% случаев ДНК ВГЧ-6 ковалентно интегрирована в субтеломерную область клеточных хромосом [5].
Интерфероны (IFN) представляют собой мультигенное семейство индуцибельных цитокинов, обладающих противовирусной активностью. Фундаментальный компонент противовирусного ответа хозяина – активация IFN I типа (IFN-α и IFN-β), который является мощным противовирусным цитокином и модулятором адаптивного иммунитета. Продукция IFN I типа индуцируется вирусной инфекцией и приводит к продукции широкого спектра противовирусных белков и иммуноактивных цитокинов. IFN-α и IFN-β продуцируются вирус-инфицированными клетками и отвечают за инициацию первичного ответа на вирусную инфекцию. IFN-α продуцируется в основном плазмоцитоидными дендритными клетками (pDCs) [6], IFN-β – различными типами клеток, включая эпителиальные и эндотелиальные клетки, фибробласты, моноциты/макрофаги и B-клетки [7]. Индукция IFN – строго регулируемый процесс, который контролируют регулирующие факторы IFN (IRF – interferon regulatory factor). Наибольшую роль в индукции IFN I типа играют IRF3 и IRF7. IRF3 конститутивно экспрессируется всеми типами клеток, тогда как экспрессию IRF7 индуцируют IFN I типа. Для длительного эффективного сохранения инфекции внутри клеток хозяина вирусы разработали множество механизмов, которые специфически нацелены на клеточные IRF, индукцию IFN и пути передачи сигналов, индуцируемых IFN [8]. IFN-γ продуцируется клетками иммунной системы, которые участвуют в раннем иммунном ответе (натуральные киллеры T-клетки и γ/δ-клетки), а также клетками иммунной системы, активированными во время адаптивных реакций (Th1 и CD8+) [9]. IFN-γ стимулирует врожденный клеточный иммунитет через NK-клетки и специфический цитотоксический иммунитет на основе распознавания связанных с клеточной поверхностью вирусных антигенов, экспрессируемых в ассоциации с белками главного комплекса гистосовместимости. Также IFN-γ активирует макрофаги, обеспечивает защиту от вирусных инфекций и длительный контроль над ними [10].
Вирусы используют сложные стратегические механизмы для предотвращения, обнаружения и разрушения иммунного ответа хозяина, включая пути антигенпрезентации, запрограммированную гибель клеток (апоптоз) и опосредованные цитокинами и хемокинами сигналы. В механизме уклонения от иммунного ответа хозяина и нарушении передачи сигналов IFN большую роль играют белки литического цикла герпесвирусов, которые являются гомологами вирусного IFN и таким образом мешают трансдукции сигнала IFN и последующей трансактивации гена [11]. Существует 5 основных способов, с помощью которых вирусы обходят ответ IFN:
- Глобальное вмешательство в экспрессию гена клетки хозяина и/или в синтез белка, включая ингибирование транскрипции клеточного гена, обработку или экспорт мРНК или синтеза клеточного белка [12].
- Минимизация индукции IFN путем ограничения продуцирования вирусных PAMP (pathogen-associated molecular patterns) и/или путем специфической блокировки каскада индукции IFN. Количество IFN, индуцированного вирусом, зависит от ряда факторов:
- количества и типа индуктора IFN, продуцируемого вирусной инфекцией. Вирусные белки, блокирующие один компонент в этой цепи, также влияют на отдаленные сигнальные или эффекторные молекулы, усиливая их ингибирующий эффект. Например, ингибиторы JAK-STAT (JAK – Janus kinase; STAT– signal transducer and activators of transcription) не только подавляют выработку противовирусных белков, но и экспрессируют IFN-индуцибельные белки, в результате чего продукция IFN «второй волны» сокращается [13];
- продукции вирусом специфического ингибитора IFN-индуцированных путей, способа его действия и кинетики синтеза. Это усиление подавления ответа IFN действует против IFN-активирующего действия молекул вирусной dsRNA, которые постепенно накапливаются в инфицированных клетках. В конечном итоге развивается баланс между вирус-стимулирующими и вирус-ингибирующими факторами, который может быть оптимальным для каждого соотношения вирус–хозяин;
- типа инфицированной клетки [14].
- Ингибирование передачи сигналов IFN. Так как между сигнальными путями для IFN I, II и III типа существуют общие компоненты, вирус способен блокировать одновременно несколько путей, то есть он может ингибировать индукцию клеточных антивирусных ферментов, не повышая экспрессии молекул главного комплекса гистосовместимости I класса в инфицированных клетках, что делает их более доступными мишенями для цитотоксических Т-лимфоцитов (ЦТЛ). Кроме того, инфицированные клетки приобретают устойчивость к воздействию IFN независимо от того, были ли IFN продуцированы инфицированными клетками или активированными лейкоцитами [15].
- Блокирование действия IFN-индуцированных ферментов с антивирусной активностью. Несколько типов вирусов, включая вирусы герпеса, кодируют белки, чтобы минимизировать активацию индуцированных IFN противовирусных белков PKR (eukaryotic initiation factor 2α kinase 2), OAS (oligoadenylate synthetase) и ADAR dsRNA (adenosine deaminases acting on viral double-stranded RNA ). Эти механизмы включают продукцию [16]: небольших, высокоструктурированных молекул РНК, которые предотвращают димеризацию PKR с помощью dsRNA и, следовательно, ее активацию; белков, непосредственно связывающихся и ингибирующих активность PKR; белков, которые являются псевдосубстратными конкурентными ингибиторами PKR; белков, разрушающих PKR (PV-протеаза); белков, индуцирующих экспрессию p58 IPK, который является клеточным ингибитором PKR; белков, косвенно влияющих на активность PKR.
- Наличие стратегии репликации, которая (в значительной степени) нечувствительна к действию IFN.
Для уклонения от ответа IFN вирусы могут использовать комбинации этих стратегий. Таким образом, если вирус блокирует передачу сигналов IFN, но не ограничивает продукции IFN, он может медленно распространяться из зараженной клетки в соседние, поскольку последние будут находиться в противовирусном состоянии, индуцированном IFN. Вероятно, из-за этого вирусы, которые блокируют передачу сигналов IFN, часто ограничивают продукцию IFN, а некоторые даже инактивируют специфические IFN-индуцированные белки с противовирусной активностью. Также имеет значение способность вирусов уменьшать продукцию любых потенциальных индукторов IFN до минимума. Это может проявляться в режиме кинетики транскрипции и репликации вируса. Любой вирусный дефект, будь то фиксация, вторжение или синтез РНК, может нарушить баланс против вируса. IFN также помогают герпесвирусам создавать и поддерживать латентность. Например, латентный мембранный белок 1 ВЭБ (LMP-1) заставляет латентно-инфицированные клетки продуцировать IFN-α/β, что помогает сохранять латентность ВЭБ двумя способами: путем предотвращения суперинфицирования клеток другими вирусами и путем ингибирования самой репликации ВЭБ [17]. IFN могут влиять на течение ВГЧ-6-инфекции, то есть инфицирование мононуклеарных клеток приводит к увеличению продукции IFN-α [18], который подавляет репликацию ВГЧ-6. Напротив, выход из клеток IFN-γ ингибируется ВГЧ-6, тогда как в Т-клетках уровень IFN-γ не изменяется после инфицирования ВГЧ-6 [18]. Результаты многочисленных исследований выявили у больных герпесвирусной инфекцией нарушение продукции IFN-α и IFN-γ, что является одним из признаков развития вторичного иммунодефицита. Вследствие этого затруднена элиминация внутриклеточного вируса, что приводит к формированию хронической рецидивирующей инфекции [19].
В последние годы опубликовано много работ, подтверждающих, что яды насекомых и животных являются богатыми источниками антимикробных веществ и содержат широкий спектр активных биологических соединений с четко выраженной химической структурой. Из гемолимфы насекомых C. vicina (Diptera, Calliphoridae) были выделены слабокатионные пептиды – аллофероны 1 и 2, состоящие из 12 и 13 аминокислотных остатков (HGVSGHGQHGVHG и GVSGHGQHGVHG соответственно). Аллоферон-2 соответствует усеченной на N-конце форме аллоферона 1. Однако непонятно, является ли присутствие двух пептидов результатом естественной деградации аллоферона-1, или молекулы аллоферона кодируются разными генами. Наибольшей активностью обладает соединение [3-13]-аллоферон-1, которое стимулирует естественную цитотоксичность лимфоцитов периферической крови человека; индуцирует продукцию IFN в эксперименте у мышей и людей; повышает противовирусную и противоопухолевую резистентность у мышей [20–22].
Аллокин-альфа – отечественный антивирусный препарат нового типа, разработанный международным коллективом ученых (рег. № 002829/01 от 22.09.2003). Действующим веществом препарата является цитокиноподобный пептид аллоферон (глистидин-глицин-валин-серин-глицин-гистидин-глицин-глутамин-гистидин-глицин-валин-гистидин-глицин).
Цель настоящего исследования – оценка влияния аллокина-альфа на уровень выделения копий ДНК ВЭБ и ВГЧ-6 в образцах слюны методом ПЦР в режиме реального времени (ПЦР-РВ), динамику продукции IFN-α и IFN-γ (спонтанного, сывороточного и индуцированного) и динамику клинических жалоб больных хронической ВЭБ- и ВГЧ-6-инфекции через месяц после окончания терапии.
Материалы и методы
Были обследованы 53 больных хронической герпесвирусной инфекцией, среди них 36 женщин и 17 мужчин. Средний возраст больных составлял 34,51 ± 1,74 года. На основании клинико-лабораторного исследования больным был поставлен диагноз «синдром хронической усталости» (СХУ) согласно критериям диагностики, опубликованным CDC (Centers for Disease Control, США) в 1988, 1991, 1992, 1994 гг. [23]. Впервые об этом заболевании сообщили американские врачи P. Cheney и D. Peterson в 1984 г., а в 1988 г. СХУ был выделен как самостоятельное заболевание [24]. В 1994 г. CDC пересмотрел зарегистрированные случаи СХУ и разработал критерии диагностики этого заболевания [23], В 2000 г. Американская коллегия ревматологов разработала и утвердила расширенную и дополненную версию единых диагностических критериев СХУ [25]. СХУ – это комплексное хроническое заболевание, оно характеризуется интенсивной, немотивируемой общей слабостью (физической и психической), которая нарушает повседневную деятельность, не уменьшается после отдыха, ухудшается при физической нагрузке и сочетается с соматическими, неврологическими, психическими и неопределенными общими расстройствами [26].
Все больные были разделены на 3 группы: в 1-ю группу вошли 26 больных с хронической ВЭБ-инфекцией (ХВЭБИ), во 2-ю – 18 больных с сочетанной инфекцией ВЭБ + ВГЧ-6, в 3-ю – 9 больных с хронической ВГЧ-6-инфекцией (ХВГЧ-6И). Длительность хронической инфекции от момента появления первых жалоб у больного до лабораторного подтверждения и постановки диагноза составила 2,82 ± 0,92 года. Ранее в течение более 6 мес. до начала обследования по назначению врача-инфекциониста большая часть больных получала противовирусную терапию препаратами из группы ациклических нуклеозидов (валацикловир, фамцикловир) без выраженного клинического эффекта. У всех больных на момент исследования отсутствовали какие-либо иммунологические нарушения или другие инфекции, а также любые хронические заболевания, которые могли повлиять на результаты исследования. В исследование не были включены больные, получавшие в течение последних 3 мес. противовирусную или иммуномодулирующую терапию.
Для ХВЭБИ, протекающей с СХУ, характерно длительное течение и частые рецидивы с клиническими и лабораторными признаками вирусной активности, подробно описанные в литературе [27–29]. Больных беспокоят длительный субфебрилитет (37,1–37,3 °С), слабость, немотивируемая утомляемость, повышенная потливость (особенно в ночное время), постоянное чувство дискомфорта и/или боли в области горла, лимфаденит, отек слизистой оболочки носа с обильным стеканием слизи, стоматит. У части больных появляется кашель, возможны кожные высыпания, артралгии, боли в мышцах туловища и конечностей. Могут возникать проявления конъюнктивита, отита. Часто развиваются неврологические расстройства: головные боли, нарушения памяти и сна, снижение концентрации внимания, раздражительность, плаксивость, склонность к депрессиям. По данным УЗИ брюшной полости, у части больных выявляется увеличение селезенки и/или печени, может беспокоить чувство тяжести в правом подреберье.
Для ХВГЧ-6И, протекающей с СХУ, характерны неврологические жалобы, такие как головные боли необычного характера или силы, головокружения, нарушение концентрации внимания, снижение памяти, слабость, утомляемость, нарушение сна, раздражительность, плаксивость, депрессивность [30]. Длительное время может держаться субфебрильная температура, наблюдаются хронические отиты, снижение остроты слуха, конъюнктивиты, алопеция (гнездная и диффузная), симптомы со стороны желудочно-кишечного тракта (боли, метеоризм, нарушения стула), сухой кашель в течение длительного времени, высыпания на коже, зуд, лимфаденит [30, 31].
Клинические методы исследования включали сбор анамнеза, данных о ранее проводимой противовирусной и иммунотерапии, наличии сопутствующих заболеваний. Клиническое состояние пациентов оценивали по общепринятой методике, включающей объективные данные и жалобы пациента на момент осмотра. Жалобы регистрировали с использованием шкалы субъективной оценки по 3-балльной шкале: 0 – отсутствие симптомов, 1 – слабая выраженность симптомов, 2 – умеренная выраженность симптомов, 3 – значительная выраженность симптомов.
Все больные получали терапию аллокином-альфа – 9 инъекций подкожно по 1,0 мг через день. Подкожное введение препарата хорошо переносилось, не вызывало аллергических реакций, не оказывало гепато-нефротоксического и токсического действия на органы кроветворения. Для оценки эффективности проводимого лечения через месяц после окончания курса терапии были проанализированы уровень выделения ДНК ВЭБ и ВГЧ-6 типа в образцах слюны, а также уровень продукции сывороточного, спонтанного и индуцированного IFN-α и IFN-γ в культуре лимфоцитов и динамика клинических жалоб.
Известно, что при хронических и атипичных формах герпесвирусной инфекции бóльшую информативность для определения ДНК вируса имеет анализ проб слюны [32], Для подтверждения вирусной этиологии заболевания у больных определяли количество ДНК ВЭБ и ВГЧ-6 в образцах слюны методом ПЦР-РВ с гибридизационно-флуоресцентной детекцией. Использовали тест-системы «АмплиСенс EBV/CMV/HHV6-скрин-FL» (ФБУН «ЦНИИ эпидемиологии», Россия).
Единицы измерения, используемые для оценки вирусной нагрузки при экстракции ДНК из слюны – количество копий ДНК ВЭБ и ВГЧ-6 на 1 мл образца (КПДНК). Согласно инструкции, этот показатель рассчитывается по формуле:
КПДНК = КДНК х 100,
где КДНК – количество копий ДНК вируса в пробе.
Аналитическая чувствительность тест-системы составляет 400 копий/ мл.
Определяли содержание IFN-α и IFN-γ в сыворотке крови, а также спонтанную и индуцированную продукцию этих цитокинов в культуре лимфоцитов крови. В качестве индуктора продукции IFN-α использовали вирус болезни Ньюкастла (NDV) (получен в ФГБУ «ГИСК им. Л.А. Тарасевича», С.-Петербург) с инфекционным титром 8 lg ЭИД/0,2 мл в объеме 8 мкл на лунку. В качестве индуктора продукции IFN-γ использовали фитогемагглютинин (ФГА-П) («ПанЭко», Россия) в дозе 10 мкг/мл. Количественное содержание цитокинов определяли в сыворотке и надосадочной жидкости 24-часовой культуры цельной крови методом твердофазного ИФА с использованием тест-систем «альфа-Интерферон-ИФА-БЕСТ» и «гамма-Интерферон-ИФА-БЕСТ» (АО «Вектор Бест», Россия). Референтные значения спонтанной, сывороточной и индуцированной продукции IFN-α и IFN-γ предоставлены производителем тест-систем.
Статистический анализ полученных результатов проводили с помощью пакета программного обеспечения IBM SPSS Statistics, версия 26 (Armonk, NY: IBM Corp.). Групповые результаты представлены в виде средней арифметической ± стандартная ошибка (М ± SD). Для сравнительной оценки результатов в группах больных использовали непараметрический U-критерий Манна–Уитни. Различия между группами считались значимыми при р ≤ 0,05.
Результаты
Из анамнеза известно, что в 1-й группе из 26 больных у 12 (46,15%) было неоднократное обострение хронического тонзиллита, 8 (30,76%) в детстве перенесли острый инфекционный мононуклеоз, 18 (69,23%) больных жаловались на частые (от 6 до 10 раз в год) ОРВИ в течение последних 8–16 мес., 24 (93,30%) связывали появление клинических жалоб с длительной стрессовой ситуацией, 10 (38,46%) длительное время проходили лечение у психотерапевта или психолога.
Во 2-й группе из 18 больных у 10 (55,56%) были частые обострения хронического тонзиллита и фарингита, трое (16,67%) в детстве перенесли острый инфекционный мононуклеоз, 10 (55,56%) больных проходили лечение у психотерапевта или психолога, 13 (72,22%) длительное время находились в состоянии хронического стресса, лечились у психотерапевта без выраженного клинического эффекта.
В 3-й группе 8 (88,89%) из 9 больных длительное время находились в состоянии хронического стресса, при этом все больные в группе в какой-то период времени проходили лечение у психотерапевта или психолога без выраженного эффекта, обращались на консультацию к психиатру. 8 (88,89%) больных жаловались на частые головные боли и головокружения, причина которых не была выявлена при обследовании у нефролога; у 6 (66,67%) больных наблюдались выраженные нарушения сна, снижение памяти и концентрации внимания; у 5 (55,56%) частые обострения хронического бронхита и трахеита сопровождались длительным (в течение нескольких месяцев) кашлем.
Результаты изучения динамики выделения ДНК ВЭБ у больных, получавших аллокин-альфа (1-я и 2-я группы), через месяц после окончания терапии представлены в табл. 1. Из данных таблицы видно, что эффективность терапии аллокином-альфа во 2-й группе ниже, чем в 1-й.
Далее был проведен анализ динамики выделения ДНК ВГЧ-6 во 2-й и 3-й группах (табл. 2). Полученные данные свидетельствуют об отсутствии влияния аллокина-альфа на уровень выделения ДНК ВГЧ-6 через месяц после окончания терапии.

Следующим этапом работы было исследование уровня продукции IFN-α и IFN-γ у больных до начала терапии и через месяц после ее окончания (табл. 3 и 4). Через месяц после курса терапии аллокином-альфа у больных во всех группах наблюдается статистически недостоверная тенденция к снижению продукции INF-α.
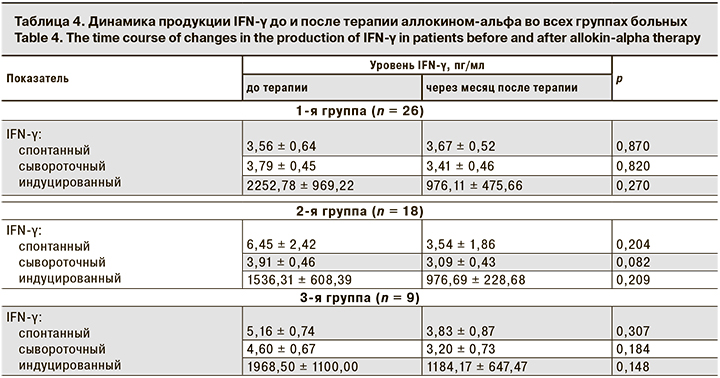
Продукция индуцированного IFN-γ у больных во всех группах имела недостоверную тенденцию к снижению через месяц после окончания терапии, показатели продукции спонтанного и сывороточного IFN-γ не имели существенной динамики после терапии.
Мы проанализировали динамику клинических жалоб в каждой группе больных через месяц после окончания проводимой терапии. В табл. 5 приведены полученные результаты.
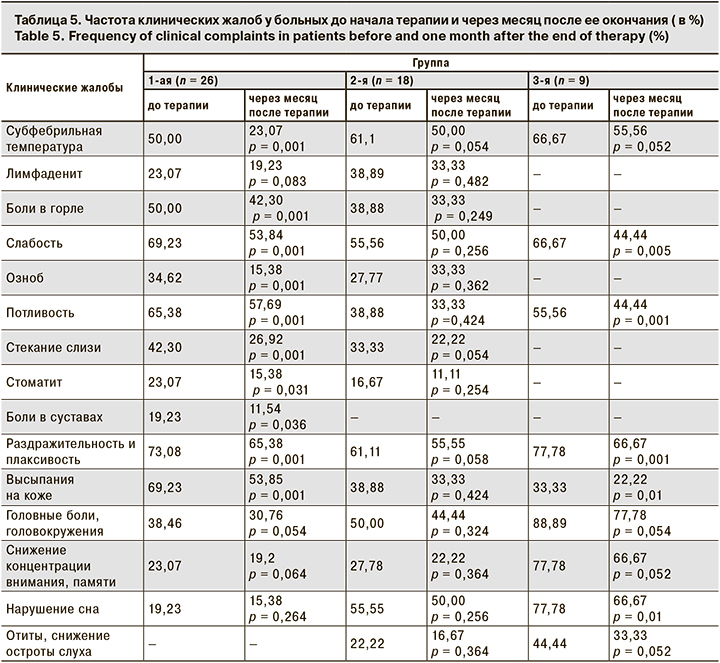
У больных 1-й группы через месяц после терапии снизилось число жалоб на субфебрильную температуру, боли в горле, слабость, озноб, потливость, стекание слизи по задней стенке глотки, стоматит, боли в суставах, раздражительность и плаксивость, высыпания на коже. Остальные жалобы остались без изменения. Во 2-й группе динамики клинических жалоб нет, отмечена только тенденция к уменьшению их числа. В 3-й группе достоверно уменьшилось число пациентов с жалобами на слабость, потливость, раздражительность и плаксивость, высыпания на коже и нарушения сна.
Обсуждение
Противогерпетический препарат должен специфически ингибировать репликацию вируса. В момент уклонения вируса от иммунного ответа хозяина он является потенциальной мишенью для химиотерапевтического воздействия. Чем выше селективность воздействия препарата, тем уже спектр его противовирусной активности, так как препараты воздействуют только на этапы репликации вируса. Несмотря на существование известных противовирусных препаратов, в мире разрабатываются все новые противовирусные соединения, которые относятся к антимикробным препаратам. Несколько катионных противовирусных пептидов были либо идентифицированы, либо синтезированы из различных источников для разработки новых терапевтических антивирусных методов лечения. Из 60 противовирусных препаратов, одобренных к настоящему времени Управлением по санитарному надзору за качеством пищевых продуктов и медикаментов (FDA – Food and Drug Administration) США, почти половина разработана для ВИЧ-1, оставшаяся половина используется для лечения вирусов гепатитов B (HBV) и C (HCV), простого герпеса (HSV), ветряной оспы (VZV), цитомегаловируса (CMV), гриппа (IAV) [33]. В настоящее время более 15 препаратов на основе пептидов находятся на разных стадиях клинических испытаний.
Действие катионных противовирусных пептидов представляет собой следующие механизмы:
- Мишени на клеточной поверхности: взаимодействие пептидов с различными гликозаминогликанами (например, HS), присутствующими на клеточной поверхности, конкурирующими с вирусом за клеточные участки связывания; блокирование проникновения вируса в клетку путем связывания пептида с вирусным CXCR4-рецептором, необходимым для его проникновения; подавление клеточного слияния путем вмешательства в активность белка АТФазы.
- Внутриклеточные мишени: подавление экспрессии вирусных генов; ингибирование удлинения пептидной цепи путем инактивации рибосомы; активация иммуномодулирующего пути путем индукции NK-клеток и IFN.
- Вирусные белки-мишени: связывание пептидов с вирусными белками, вызывающими ингибирование адсорбции/слияния вирусных клеток [34].
Аллоферон представляет собой иммуномодулирующий пептид из 13 аминокислот, физиологическая роль которого в организме хозяина до конца не известна. Препарат был впервые выделен из иммунной системы насекомых [35]. Он обладает противоопухолевым эффектом посредством повышения активности NK-клеток и антивирусным эффектом, особенно против вируса герпеса, ассоциированного с саркомой Капоши (ВГЧ-8) и механизмами регуляции жизненного цикла вируса; повышает киллерную активность NK-клеток в отношении опухолевых клеток посредством усиления экспрессии NK-активирующих рецепторов 2B4; увеличивает продукцию IFN-γ и TNF-α и гранулированный экзоцитоз гранул из NK-клеток против опухолевых клеток [36, 37]; эффективно ингибирует продукцию провоспалительных цитокинов, таких как IL-6, IL-8 и TNF-α при УФ-индуцированном воспалении кожи [38].
В нашем исследовании показано, что количество копий ДНК ВЭБ в образцах слюны в 1-й и 2-й группах через месяц после окончания терапии аллокином-альфа различается. В 1-й отмечена высокая чувствительность к препарату, что подтверждено отрицательными результатами ПЦР ДНК ВЭБ в образцах слюны (00,00 копий в 1 мл образца) у 15 (57,69%) больных. Во 2-й группе отрицательные результаты были выявлены у 8 (44,4%) больных. Мы предпологаем, что это может быть обусловлено способностью ВГЧ-6 активировать репликацию ВЭБ за счет увеличения экспрессии раннего гена Zebra ВЭБ в 10 раз, что приводит к последующей репликации ВЭБ.
Анализ динамики выделения ДНК ВГЧ-6 в образцах слюны во 2-й и 3-й группах показал, что во 2-й группе отрицательное значение ПЦР ДНК ВГЧ-6 (00,00 копий в 1 мл образца) было выявлено только у 1 (5,55%) больного. В 3-й группе не было получено ни одного отрицательного результата. Это подтверждает данные литературы об отсутствии специфических противовирусных препаратов для лечения ВГЧ-6-инфекции [39].
В 2002 г. были опубликованы результаты изучения индукции продукции IFN-α и IFN-γ под влиянием аллоферона in vivo и in vitro [35]. Авторы показали, что ранний синтез IFN, индуцированный введением аллоферона, может стимулировать вторичный ответ, превышающий первичный в тех же или других IFN-продуцирующих клетках, отражая тем самым каскадоподобную реакцию. В работе Н.В. Коноваловой Н.В. и соавт. [40] у больных увеитами вирусной этиологии (ВПГ-1) отмечено увеличение продукции IFN-α в группе, получавшей терапию аллокином-альфа, с 12,29 ± 6,367 до 19,24 ± 11,47 пг/мл (р = 0,009) и увеличение продукции IFN-γ с 19,95 ± 7,778 до 28,81 ± 10,71 пг/мл (р = 0,013). Однако авторы проводили исследование сразу после окончания терапии и не представили более отдаленных результатов. Н.В. Зароченцева и соавт. [41] опубликовали отдаленные результаты исследования продукции IFN-α и IFN-γ у пациенток с хроническим эндометритом и привычным невынашиванием беременности после терапии аллокином-альфа. Авторы показали, что через 2 мес. после окончания лечения происходит увеличение продукции IFN-α и IFN-γ, но не представили абсолютных значений показателей, что не дает точной информации о динамике продукции IFN. В работе С.И. Черныш [42] показано, что концентрация IFN повышается через 2 ч после введения аллокина-альфа и сохраняется на высоком уровне (в 2–2,5 раза выше обычного фонового) на протяжении 6–8 ч, снижаясь до исходных значений к концу суток. Автор делает вывод о том, что препарат действует как индуктор IFN. Результаты нашего исследования показали, что продукция IFN-α (сывороточная, спонтанная) достоверно не изменяется через месяц после терапии аллокином-альфа. Уровень индуцированного IFN-α имел тенденцию к снижению. Продукция IFN-γ (сывороточного, спонтанного, индуцированного) во всех группах больных также имела недостоверную тенденцию к снижению через месяц после окончания терапии. Мы не определяли уровень продукции IFN через 2 ч после введения препарата, поэтому не можем подтвердить результаты, ранее полученные С.И. Черныш.
Однако мы предположили, что может быть еще один механизм снижения продукции IFN за счет стратегии уклонения вируса от ответа. IFN индуцируют синтез нескольких противовирусных белков, которые служат клеточно-автономными внутренними факторами рестрикции. Для преодоления внутриклеточных ограничений вирулентные вирусы ингибируют синтез IFN, связывают и инактивируют секретируемые молекулы IFN, блокируют IFN-активированную передачу сигналов или нарушают действие IFN-индуцированных антивирусных белков. Многие вирусы продуцируют специализированные белки, которые разрывают сигнал опасности или экспрессию вирулентных генов, нацеленных на членов семейства регуляторных факторов IFN (IRF) или компонентов сигнального пути JAK-STAT. Альтернативная стратегия уклонения вирусов основана на экстремальной скорости репликации вируса, которая превосходит ответ IFN [43]. Вероятно, вирусы и врожденный иммунный ответ хозяина эволюционировали таким образом, что развился тонкий баланс между вирусными и вирус-ингибирующими факторами, то есть в оставшейся части ВЭБ- и ВГЧ-6-инфицированных клеток вирус сохраняется в репликативной фазе, следовательно, сохраняется механизм подавления продукции IFN. Именно поэтому уровень продукции IFN-α и IFN-γ после терапии аллокином-альфа не имеет достоверной тенденции к увеличению.
Анализ клинических жалоб показал достоверную положительную динамику во всех 3 группах после терапии аллокином-альфа. Больные особенно отмечали улучшение общего физического состояния, повышение работоспособности, появления «желания жить», чего не происходило на фоне проводимой ранее стандартной противовирусной терапии ациклическими нуклеозидами. Введение препарата хорошо переносилось больными.
Полученные нами результаты свидетельствуют о высокой эффективности аллокина-альфа в терапии взрослых с СХУ на фоне ХВЭБИ и отсутствии противовирусного эффекта у больных с СХУ на фоне ХВГЧ-6И.
Выводы
- Аллокин-альфа оказывает выраженный эффект на уровень выделения ДНК ВЭБ в образцах слюны у больных ХВЭБИ через месяц после окончания терапии.
- Противовирусный эффект препарата менее выражен у больных с сочетанной герпесвирусной инфекцией (ВЭБ + ВГЧ-6) по сравнению с больными ХВЭБИ.
- Применение препарата не вызывает достоверного снижения уровня ДНК ВГЧ-6 у больных ХВГЧ-6И через месяц после окончания терапии.
- Аллокин-альфа не приводит к достоверному увеличению продукции IFN-α и IFN-γ через месяц после окончания терапии.
- У больных с СХУ на фоне герпесвирусной инфекции через месяц после окончания терапии аллокином-альфа отмечается уменьшение числа клинических жалоб.
- Препарат может быть рекомендован для лечения пациентов с СХУ на фоне герпесвирусной инфекции (ВЭБ, ВЭБ + ВГЧ-6 и ВГЧ-6) в дозе 1 мг подкожно через день при курсовой дозе не менее 9 инъекций.


