COVID-19 and the risk of herpesvirus reactivation
DOI: https://dx.doi.org/10.18565/epidem.2021.11.2.55-62
Solomay T.V., Semenenko T.A., Isaeva E.I., Vetrova E.N., Chernyshova A.I., Romenskaya E.V., Karazhas N.V.
1) Interregional Department One, Federal Biomedical Agency of Russia, Moscow, Russia;
2) I.I. Mechnikov Research Institute of Vaccines and Sera, Ministry of Education and Science of Russia, Moscow, Russia;
3) Honorary Academician N.F. Gamaleya National Research Center for Epidemiology and Microbiology, Ministry of Health of Russia, Moscow, Russia;
4) I.M. Sechenov First Moscow State Medical University (Sechenov University), Ministry of Health of Russia, Moscow, Russia;
5) N.N. Burdenko Main Military Clinical Hospital, Ministry of Defense of Russia, Khimki, Moscow Region, Russia
Objective. To study the features of the epidemic process of infections caused by herpes simplex virus types 1 and 2 (HSV-1, HSV-2), Epstein-Barr virus (EBV), cytomegalovirus (CMV), and human herpes virus type 6 (HHV6) during the COVID-19 pandemic.
Materials and methods. The incidence of COVID-19 and recorded herpesvirus infections was retrospectively analyzed. Ninety-two blood donors and 95 COVID-19 patients without respiratory failure were examined for herpes virus infection markers.
Results. There was an earlier and longer surge in the incidence of infectious mononucleosis in 2020 versus 2019; there were no significant differences between the groups of patients and donors in the detection rate of IgG to HSV-1 (88–91.6%), HSV-2 (20–20.7%), EBV (98.9–100%), CMV (82.1–83.7%), HHV6 (48.4–51.1%); low-avidity IgG to HSV (6.5–8.4%); EBV (2.2–6.3%) and CMV (0–1.1%); IgM to HSV-1 (0%), HSV-2 (0–1.1%,), CMV (0–2.2%), and HHV6 (5.4–8.4%). EBV reactivation markers (VCA IgM and EA IgG in the presence of VCA IgG and EBNA IgG) were significantly more frequently detected in patients (70.5% and 56.8%) than in donors (0 and 2.2%) (p < 0.05).
Conclusion. SARS-CoV-2 is a factor that triggers the mechanism of EBV transition from latency to lytic reproduction in the human body, whereas COVID-19 patients are at risk for reactivation of chronic EBV infection.
По мнению ряда авторов, бремя герпесвирусных инфекций (ГВИ)_на настоящий момент является недостаточно оцененным [1, 2]. Известны 9 представителей этого семейства, вызывающих заболевание у человека: вирусы простого герпеса 1-го и 2-го типов (ВПГ-1 и ВПГ-2), Varicella-Zoster virus (VZV), вирус Эпштейна–Барр (ВЭБ), цитомегаловирус (ЦМВ), вирусы герпеса человека 6-го (ВГЧ-6А и ВГЧ-6В), 7-го и 8-го типов (ВГЧ-7 и ВГЧ-8) [3, 4]. Однако методы рутинной лабораторной диагностики разработаны и внедрены в практику только для ВПГ-1, ВПГ-2, VZV, ВЭБ, ЦМВ, ВГЧ-6 без подразделения на ВГЧ-6А и -6В [5]. В ряде стран мира, в том числе в Российской Федерации, официальной регистрации подлежат только случаи ветряной оспы (опоясывающего лишая), инфекционного мононуклеоза и цитомегаловирусной инфекции (ЦМВИ) [6].
В то же время широкая повсеместная распространенность и хронический характер течения ГВИ определяют возможность развития сочетанной патологии практически с любым патогеном [7–12]. В литературе описаны клинические проявления простого герпеса и инфекции, вызванной VZV, на фоне гриппа [13, 14], кори [15, 16], ВИЧ-инфекции [17] и др. Как правило, сочетанные инфекции имеют более тяжелое клиническое течение.
Пандемия новой коронавирусной инфекции COVID-19 способствовала переоценке роли ГВИ. Особое внимание исследователей привлекли случаи развития опоясывающего лишая (VZV) на фоне инфекции, вызванной SARS-CoV-2. Высказано предположение, что реактивация латентной VZV-инфекции, вызвана воспалительной реакцией при COVID-19 в носоглотке [18]. Установлено, что перед появлением клинических признаков опоясывающего лишая у пациентов, инфицированных SARS-CoV-2, происходит снижение количества CD3+- и CD8+-лимфоцитов. В среднем высыпания, характерные для VZV, появлялись на 4–7-й день от момента госпитализации в стационар больных с коронавирусной инфекцией на фоне приема гидроксихлорохина и не имели связи с проведением искусственной вентиляции легких [19]. В другом исследовании показано, что клинические проявления опоясывающего лишая имеют место даже после перенесенной субклинической SARS-CoV-2-инфекции [20].
В то же время довольно незначительное число публикаций посвящено вопросу микст-инфицирования SARS-CoV-2 с ВПГ-1, ВПГ-2, ВЭБ, ЦМВ и ВГЧ-6 [21 31]. Описанные единичные клинические случаи не позволяют установить частоту развития активной ГВИ на фоне COVID-19. В этой связи особый интерес представляет изучение эпидемиологических особенностей ГВИ на раннем этапе развития пандемии COVID-19 (весна 2020 г.) путем проведения исследования, которое позволит ответить на вопрос: являются ли пациенты с COVID-19 группой риска развития активной инфекции, вызванной вирусами герпеса?
Целью исследования явилось изучение особенностей развития эпидемического процесса инфекций, вызванных ВПГ-1, ВПГ-2, ВЭБ, ЦМВ и ВГЧ-6 на фоне развития пандемии COVID-19.
Для этой цели предполагалось:
1. Проанализировать внутригодовую динамику заболеваемости населения Москвы COVID-19 в 2020 г. и регистрируемыми нозологическими формами ГВИ (инфекционный мононуклеоз и ЦМВИ) в 2019 и 2020 гг.
2. Сопоставить частоту выявления серологических маркеров инфицирования вирусами герпеса (ВПГ-1, ВПГ-2, ВЭБ, ЦМВ, ВГЧ-6) в группах больных COVID-19 и условно здоровых лиц.
Материалы и методы
Проведен ретроспективный анализ заболеваемости населения Москвы COVID-19 и регистрируемыми нозологическими формами ГВИ (инфекционный мононуклеоз и ЦМВИ). Материалами послужили данные формы № 2 Росстата «Сведения об инфекционных и паразитарных заболеваниях» за 2019 и 2020 гг. Информация о числе заболевших COVID-19 за 2020 г. получена из общедоступного интернет-ресурса «Стопкоронавирус.рф»
Весной 2020 г. (март–май) на наличие серологических маркеров инфицирования вирусами герпеса (ВПГ-1, ВПГ-2; ВЭБ; ЦМВ; ВГЧ6) в крови было обследовано 92 условно здоровых человека (доноры крови и ее компонентов, далее – доноры) и 95 пациентов с COVID-19 без дыхательной недостаточности с впервые в жизни установленным диагнозом. У 30 пациентов заболевание протекало в виде острого назофарингита, у 65– интерстициальной пневмонии, не требующей проведения искусственной вентиляции легких. Все пациенты находились на лечении в инфекционном стационаре для больных COVID-19.
Из 95 обследованных РНК SARS-CoV-2 в мазках из носоглотки была выявлена только у 59 чел. (62,1%; 95% ДИ 57,1–67,1). Антитела к SARS-CoV-2 (IgM, IgG или их сочетание) на 10–21-й день от начала клинических проявлений COVID-19 выявлены у 86 пациентов (90,5%; 95% ДИ 84,6–96,4). У 36 чел. с отрицательными результатами исследования мазка из носоглотки на выявлены Ig к SARS-CoV-2, в том числе только IgM – у 4 пациентов, IgG – у 8, сочетание IgM и IgG – у 24. У 8 пациентов с выявленными IgG данные о заболевании COVID-19 до настоящей госпитализации в анамнезе отсутствовали. Таким образом, у всех пациентов инфекция COVID-19 подтверждена лабораторно.
Группы доноров и пациентов были сопоставимы по полу и возрасту. В группе доноров женщины составили 33,7% (95% ДИ 23,79–43,61), мужчин – 66,3% (95% ДИ 56,39–76,21); в группе пациентов –16,8% (95% ДИ 9,2–24,4) и 83,2% (95% ДИ 75,6–90,8) соответственно. Средний возраст обследованных доноров – 41,0 год (95% ДИ 38,8–43,2), пациентов – 42,4 года (95% ДИ 33,6–51,2).
Критерии включения в исследование:
- возраст от 18 до 65 лет (мужчины и женщины);
- постоянное (на протяжении 10 и более лет) проживание на территории Москвы или Московской области;
- отсутствие алкогольной и/или наркотической зависимости;
- отсутствие психических расстройств, требующих лечения антидепрессантами;
- отсутствие идентифицированных маркеров ВИЧ, гепатитов В и С;
- отсутствие иной инфекционной и соматической патологии, сопровождающейся иммунодефицитом, или приема лекарственных препаратов, относящихся к группе иммунодепрессантов;
- наличие информированного согласия на проведение лабораторно-инструментального обследования.
В исследование не были включены:
- доноры, не допущенные по медицинским показаниям к сдаче крови и ее компонентов;
- доноры, имеющие в анамнезе COVID-19 или антитела к SARS-CoV-2;
- пациенты, ранее перенесшие COVID-19, у которых госпитализация по поводу данной инфекции является повторной;
- пациенты, у которых наличие COVID-19 не подтверждено выделением РНК SARS-CoV-2 методом ОТ-ПЦР РВ в мазке из носоглотки или обнаружением антител (IgM и/или IgG) методом ИФА;
- пациенты с тяжелым клиническим течением COVID-19, требующие проведения искусственной вентиляции легких.
Из исследования также исключали лиц, сыворотки крови от которых имели признаки гемолиза.
Забор крови для исследования у доноров проводили непосредственно перед донацией. У пациентов с COVID-19 взятие мазка из носоглотки проводили в день первичного обращения за медицинской помощью, забор крови – на 10–21-й день от начала клинических проявлений.
Исследования на наличие IgM и IgG к ВПГ-1 и ВПГ- 2, ВЭБ (IgM VCA, IgG VCA, IgG EA, IgG EBNA), ЦМВ, SARS-CoV-2; IgG – к ВГЧ-6, определение авидности IgG ВПГ-1, ВПГ-2, а также ЦМВ и ВЭБ (IgG VCA) проводили методом ИФА с использованием наборов реагентов производства АО «Вектор-Бест» (Новосибирск, Россия). IgM к ВГЧ-6 определяли в непрямой реакции иммунофлюоресценции с помощью набора реагентов Euroimmun AG (Германия). Результаты оценивали в соответствии с критериями, изложенными в инструкциях.
Для статистической обработки результатов использовали таблицы Microsoft Excel, 2019. Рассчитывали показатели заболеваемости на 100 тыс. населения, частоты выявления серологических маркеров в % и их доверительные интервалы. Различия считались достоверными при доверительной вероятности 95% и доверительной значимости (p) < 0,05. Для изучения синхронности внутригодовой динамики заболеваемости инфекционным мононуклеозом, ЦМВИ и COVID- 19 использовали коэффициент линейной корреляции Пирсона (r). При положительном значении r связь расценивалась как прямая, при отрицательном – как обратная. Модуль r указывал на силу связи: от 0 до 0,29 – слабая, от 0,3 до 0,69 – средняя, от 0,7 до 1 – сильная. Для оценки достоверности показателя r рассчитывали его ошибку (mr) и коэффициент Стьюдента (t). Различия считались достоверными при доверительной вероятности 95% и p < 0,05.
Результаты
Первые случаи COVID-19 в Москве были зарегистрированы в марте 2020 г. Последующий рост числа заболевших в апреле и мае происходил на фоне интенсивного снижения заболеваемости инфекционным мононуклеозом и менее выраженного – ЦМВИ (см. рисунок). С июля по декабрь в динамике заболеваемости COVID-19 и инфекционным мононуклеозом зарегистрирована синхронная тенденция к росту (r = 0,9, mr = 0,05, t = 18; p < 0,05 – связь достоверная сильная прямая). Между показателями заболеваемости ЦМВИ и COVID-19 за аналогичный период установлена прямая корреляционная связь средней силы (r = 0,6, mr = 0,18, t = 3,3; p < 0,05 – связь достоверная прямая средней силы).
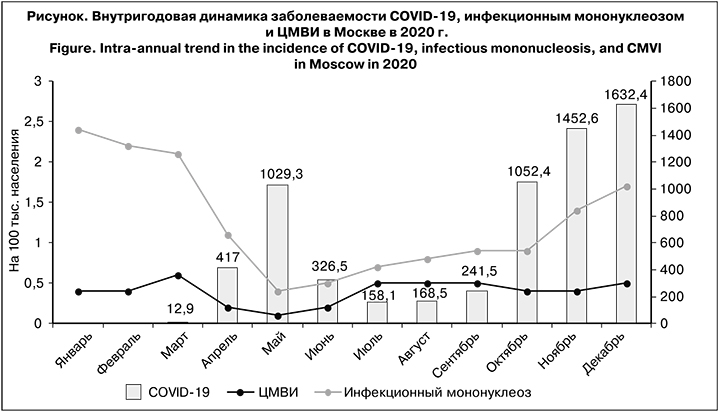
При сравнении заболеваемости инфекционным мононуклеозом и ЦМВИ в 2020 и 2019 гг. установлено.что в 2019 г. показатели заболеваемости ЦМВИ (6,3 на 100 тыс. населения; 95% ДИ 6,1–6,5) и инфекционным мононуклеозом (24,7 на 100 тыс.; 95% ДИ 23,5–25,9) были достоверно выше, чем в 2020 г. – 4,7 (95% ДИ 4,5–4,9) и 15,1 (95% ДИ 14,4–15,8) на 100 тыс. соответственно (р < 0,05).
 Внутригодовая динамика заболеваемости инфекционным мононуклеозом характеризовалась сезонным подъемом в холодный период года и относительным эпидемическим благополучием в летние месяцы, которое в 2019 г. продолжалось с мая по август, а в 2020 г. после минимального показателя в мае (0,4 на 100 тыс. населения) сразу же был отмечен рост. Изменение заболеваемости ЦМВИ по месяцам в 2020 г. существенно не отличалось от такового в 2019 г. Как в 2019, так и в 2020 гг. выраженный сезонный подъем показателей не выявлен.
Внутригодовая динамика заболеваемости инфекционным мононуклеозом характеризовалась сезонным подъемом в холодный период года и относительным эпидемическим благополучием в летние месяцы, которое в 2019 г. продолжалось с мая по август, а в 2020 г. после минимального показателя в мае (0,4 на 100 тыс. населения) сразу же был отмечен рост. Изменение заболеваемости ЦМВИ по месяцам в 2020 г. существенно не отличалось от такового в 2019 г. Как в 2019, так и в 2020 гг. выраженный сезонный подъем показателей не выявлен.
Исследование частоты выявления серологических маркеров инфицирования ВПГ-1, ВПГ-2, ВЭБ, ЦМВ и ВГЧ-6 проведено на фоне роста заболеваемости COVID-19 (первая волна) и снижения – регистрируемыми нозологическими формами ГВИ.
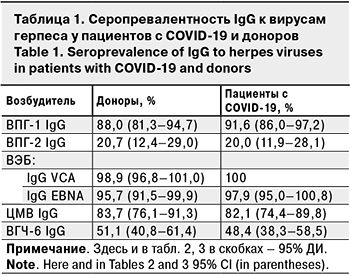
Оценка серопревалентности IgG к ВПГ-1, ВПГ-2, ВЭБ, ЦМВ и ВГЧ-6 не выявила достоверных различий между группами пациентов и доноров (табл. 1).
Дополнительное определение индекса авидности IgG к ВПГ-1, ВПГ-2, ВЭБ и ЦМВ также не позволило выявить значимых различий между группами (табл. 2). Расчет удельного веса низкоавидных IgG на 100 обследованных дает представление о частоте выявления первичной острой инфекции во взрослой популяции.

Частота выявления серологических маркеров активной ГВИ – IgM к ВПГ-1, ВПГ-2, ЦМВ и ВГЧ-6 – в исследуемых группах была сопоставима (табл. 3). Исключение составили маркеры активной ВЭБ-инфекции – IgM к капсидному (IgM VCA) и IgG к раннему (IgG EA) антигенам, которые достоверно чаще выявляли в группе пациентов (p < 0,05).
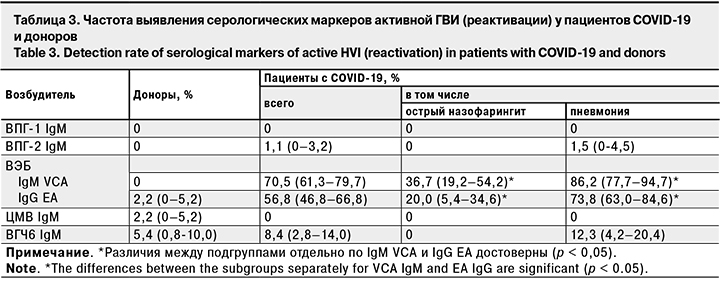
Анализ результатов обследования на маркеры активной инфекции пациентов с острым назофарингитом и интерстициальной пневмонией без дыхательной недостаточности выявил достоверное превышение частоты выявления IgM VCA и IgG EA к ВЭБ и IgM к ВГЧ-6 у последних (p < 0,05).
Обсуждение
Первая волна эпидемического процесса COVID-19 весной 2020 г. совпала с сезонным снижением заболеваемости инфекционным мононуклеозом и, по всей видимости, спровоцировала в дальнейшем ее более ранний и продолжительный подъем с июня по декабрь включительно, по сравнению с 2019 г. (с сентября по ноябрь). По результатам другого исследования для инфекционного мононуклеоза в Москве в 2014–2018 гг. установлены сезонные колебания показателей с периодом относительного эпидемического благополучия в июне–августе [32]. Таким образом, именно 2020 г. характеризуется выраженными изменениями внутригодовой динамики заболеваемости инфекционным мононуклеозом. Схожих тенденций в заболеваемости ЦМВИ не выявлено.
Выдвинутая гипотеза о влияния COVID-19 на эпидемический процесс инфекционного мононуклеоза, этиологическим агентом которого в 90% случаев является ВЭБ, подтверждается не только наличием сильной прямой корреляционной связи между изменениями показателей заболеваемости этими нозологиями, но и результатами проведенных серологических исследований.
Было показано, что на фоне сопоставимых показателей серопревалентности к ВПГ-1, ВПГ-2, ВЭБ, ЦМВ и ВГЧ-6, частоты выявления низкоавидных IgG к ним, а также маркеров, указывающих на реактивацию этих возбудителей в группах доноров и пациентов с COVID- 19, основным отличием стал показатель выявления маркеров реактивации ВЭБ-инфекции (IgM VCA и IgG EA на фоне присутствия IgG VCA и IgG EBNA), который у пациентов был достоверно выше.
Вероятной причиной реактивации ВЭБ-инфекции может быть описанное в литературе изменение иммунологической реактивности организма человека под действием COVID-19, проявляющееся дисбалансом CD4+ и CD8+ T-клеток [12, 33]. С другой стороны, ранее было показано, что реактивация ВЭБ в культуре клеток происходит без участия иммунной системы хозяина под влиянием внешних негативных факторов [34]. В этой связи не исключено, что SARS-CoV-2 может оказывать непосредственное воздействие на клетки, латентно инфицированные вирусами герпеса, вступая с ними в конкурентные отношения и, тем самым, вызывая реактивацию. В каждом из 2 предполагаемых сценариев SARS-CoV-2 создает благоприятные условия к переходу вирусов герпеса из латентного состояния к репродукции.
Установленная в настоящем исследовании частота выявления низкоавидных IgG -маркеров первичной инфекции, вызванной ВПГ-1, ВПГ-2, ВЭБ и ЦМВ у пациентов с COVID-19 (8,4, 6,3 и 1,1 на 100 обследованных соответственно) и доноров (6,5, 2,2, 0) согласуется с данными других исследований, в ходе которых показано, что у взрослого населения низкоавидные IgG к ВПГ-1 и ВПГ-2 обнаруживают в 0,7–5,1% случаев [35– 37], ВЭБ – в 3,6–4,2% [9], ЦМВ – в 2,0% [36].
Не было выявлено особенностей и в уровнях серопревалентности к ВПГ-1, ВПГ-2, ВЭБ, ЦМВ и ВГЧ-6 у пациентов с COVID-19 (91,6, 20,0, 100, 82,1 и 48,4% соответственно), которые не имели достоверных отличий от таковых у доноров (88,0, 20,7, 98,9, 83,7 и 51,1%). Для сравнения: серопревалентность к ВПГ-1, ВПГ- 2, ВЭБ, ЦМВ и ВГЧ-6 среди разных групп взрослого населения, по данным других исследований, составляет 59,3–82,4% [38, 39], 21,1–51,8% [38, 39], 72–99,5% [40, 41], 51–100% [42, 43], 51–90% [11, 44] соответственно.
Это свидетельствует в пользу отсутствия влияния особенностей предшествующего развития эпидемического процесса ВЭБ-инфекции на заболеваемость COVID-19 и еще раз подтверждает триггерную роль SARS-CoV-2 в реактивации этой ГВИ.
В литературе описаны единичные случаи реактивации ВЭБ на фоне COVID-19 [31, 45]. При этом наибольший интерес представляет исследование, в ходе которого ДНК ВЭБ в крови была выявлена у 95,2% пациентов с тяжелым течением COVID-19, находящихся на искусственной вентиляции легких, и у 83,6% пациентов без ИВЛ. Важно отметить, что генетический материал других герпесвирусов (ЦМВ и ВГЧ-6) обнаружен не был [46, 47]. Исследование на наличие серологических маркеров ГВИ в группах пациентов с COVID-19 и здоровых лиц на репрезентативной выборке, позволяющее дифференцировать первичную острую инфекцию и реактивацию, ранее не проводилось и было выполнено нами впервые.
Анализ публикаций показывает, что интерес ученых был прикован к пациентам с тяжелыми формами COVID-19, частота выявления активной ГВИ у больных с легким и среднетяжелым течением без дыхательной недостаточности ранее не оценивалась. Тем не менее, проведенное нами сопоставление показателей в подгруппах пациентов с легким и среднетяжелым течениемCOVID-19, впервые позволило установить зависимость частоты выявления IgM VCA и IgG EA к ВЭБ и IgM к ВГЧ-6 от тяжести заболевания.
Таким образом, в настоящем исследовании представлены убедительные доказательства роли SARS-CoV-2 в реактивации ВЭБ на 10–21-е сутки от момента обращения пациентов за медицинской помощью, что дает основание для дальнейшего углубленного изучения этой проблемы.
Выводы
1. Выявлены особенности эпидемического процесса ГВИ в Москве на фоне развития пандемии COVID-19 весной 2020 г.:
- высокая распространенность серопозитивности к инфекциям, вызванным ВПГ-1, ВПГ-2, ВЭБ, ЦМВ и ВГЧ-6, как среди пациентов с COVID-19, так и среди здоровых лиц (88–91,6, 20–20,7, 98,9–100, 82,1–83,7 и 48,4–51,1% соответственно);
- равнозначная частота выявления маркеров первичной острой инфекции, вызванной ВПГ-2 и ВПГ-2 (6,5–8,4%), ВЭБ (2,2–6,3%), ЦМВ (0–1,1%) и реактивации ВПГ-1, ВПГ-2, ЦМВ и ВГЧ-6 (0, 0–1,1, 0–2,2 и 5,4–8,4% соответственно) в группах доноров и пациентов с COVID-19 (p > 0,05);
- более ранний и продолжительный подъем во внутригодовой динамике заболеваемости инфекционным мононуклеозом с июня по декабрь включительно по сравнению с 2019 г;
- достоверно более высокая частота реактивации ВЭБ-инфекции (наличие IgM VCA и IgG EA на фоне IgG VCA и IgG EBNA) в группе пациентов с COVID-19 по сравнению с донорами (70,5 и 56,8% против 0 и 2,2% соответственно; p < 0,05).
2. Согласно полученным данным, SARS-CoV-2 можно рассматривать как триггерный фактор, запускающий в организме человека механизм перехода ВЭБ от фазы латенции к литической репродукции, а пациентов COVID-19 – как группу риска по реактивации хронической ВЭБ-инфекции.
Literature
1. Johnson R.W., Bouhassira D., Kassianos G., Leplège A., Schmader K.E., Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010; (8): 37. doi: 10.1186/1741-7015-8-37
2. Damm O., Witte J., Wetzka S., Prosser C., Braun S., Welte R., Greiner W. Epidemiology and economic burden of measles, mumps, pertussis, and varicella in Germany: a systematic review. Int. J. Public. Health 2016; 61(7): 847–60. doi: 10.1007/s00038-016-0842-8
3. Ablashi D., Agut H., Alvarez-Lafuente R., Clark D.A., Dewhurst S., DiLuca D. et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch. Virol. 2014; 159(5): 863–70. doi: 10.1007/s00705-013-1902-5
4. Мелехина Е.В., Лысенкова М.Ю., Свитич О.А., Музыка А.Д., Каражас Н.В., Рыбалкина Т.Н. и др. Особенности течения инфекции ВГЧ-6А и ВГЧ-6В у детей, проживающих в Московском регионе. Эпидемиол. инфекц. болезни. Актуал. вопр. 2018; (2): 42–9. doi: 10.18565/epidem.2018.2.42-9
Melehina E.V., Lysenkova M.Yu., Svitich O.A., Muzyka A.D., Karazhas N.V., Rybalkina T.N. et al. [Features of the course of HHV-6A and HHV-6B infection in children living in the Moscow Region]. Èpidemiologiâ i infekcionnye bolezni. Аktual’nye voprosy 2018; (2): 42–9. (In Russ.). doi: 10.18565/epidem.2018.2.42-9
5. Соломай Т.В., Семененко Т.А., Каражас Н.В., Рыбалкина Т.Н., Корниенко М.Н., Бошьян Р.Е. и др. Оценка риска инфицирования герпесвирусами при переливании донорской крови и ее компонентов. Анализ риска здоровью 2020; (2): 135–42 doi: 10.21668/health.risk/2020.2.15.eng
Solomay T.V., Semenenko T.A., Karazhas N.V., Rybalkina T.N., Kornienko M.N., Bosh’yan R.E. et al. [Assessing risks of infection with herpes viruses during transfusion of donor blood and its components]. Health Risk Analysis 2020; (2): 135–42. (In Russ.). doi: 10.21668/health.risk/2020.2.15.eng
6. Соломай Т.В., Семененко Т.А. Вирусные гепатиты В, С и инфекционный мононуклеоз: эпидемиологическое сходство и различия. Вопросы вирусологии 2020; 65(1): 27–34 doi: https://doi.org/10.36233/0507-4088-2020-65-1-27-34
Solomay T.V., Semenenko T.A. [Viral hepatitis B, C and infectious mononucleosis: epidemiological similarities and differences]. Voprosy virusologii 2020; 65(1): 27–34. (In Russ.). doi: 10.36233/0507-4088-2020-65-1-27-34 (in Russian)
7. Sharifipour S., Davoodi Rad K. Seroprevalence of Epstein–Barr virus among children and adults in Tehran, Iran. New Microbes New Infect. 2020; 34: 100641. doi: 10.1016/j.nmni.2019.100641
8. Cui J., Yan W., Xu S., Wang Q., Zhang W., Liu W., Ni A. Anti-Epstein–Barr virus antibodies in Beijing during 2013<2017: What we have found in the different patients. PLoS One 2018; 13(3): e0193171. doi: 10.1371/journal.pone.0193171
9. Beader N., Kolarić B., Slačanac D., Tabain I., Vilibić-Čavlek T. Seroepidemiological Study of Epstein–Barr Virus in Different Population Groups in Croatia. Isr. Med. Assoc. J. 2018; 20(2): 86–90.
10. Smatti M.K., Yassine H.M., Abu Odeh R., Al Marawani A., Taleb S.A., Althani A.A., Nasrallah G.K. Prevalence and molecular profiling of Epstein–Barr virus (EBV) among healthy blood donors from different nationalities in Qatar. PLoS One 2017; 12(12): e0189033. doi: 10.1371/journal.pone.0189033
11. Соломай Т.В., Семененко Т.А., Каражас Н.В., Рыбалкина Т.Н., Веселовский П.А., Пульнова Н.Л. и др. Особенности изменения показателей иммунного статуса лиц с активными и латентными формами герпесвирусных инфекций. Пермский медицинский журнал.2021; 38 (1): 46–63. doi: 10.17816/pmj38146%63
Solomay T.V., Semenenko T.A., Karazhas N.V., Rybalkina T.N., Veselovsky P.A., Pulnova N.L. et al. [Features of changes in the immune status of individuals with active and latent forms of herpesvirus infections]. Permskij medicinskij zhurnal.2021; 38 (1): 46–63. (In Russ.). doi: 10.17816/pmj38146%63
12. Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L. et al. Characteristics of Peripheral Lymphocyte Subset Alterationin COVID-19 Pneumonia. J. Infect. Dis. 2020; 221(11): 1762–9. doi: 10.1093/infdis/jiaa150
13. Matsubara H., Konishi T., Saito K., Naito A., Sugisawa J., Nakayama S. et al.. Zoster duplex in a patient with influenza a and bacterial superinfection. J. Dermatol. 2020; 47(1): e32–e33. doi: 10.1111/1346-8138.15099
14. Pereiro T., Lourido T., Ricoy J., Valdés L. Influenza Virus, Herpes Simplex Virus and Methicillin-Resistant Staphylococcus aureus in an Immunocompetent Patient. Arch. Bronconeumol. 2018; 54(3): 159–60. doi: 10.1016/j.arbres.2017.07.005
15. Li C., Li Y., Yang Y., Wang J., Zhu C., Tang S. et al. The Detection and Characterization of Simplex Virus Type 1 in Confirmed Cases. Sci. Rep. 2019; 9(1): 12785. doi: 10.1038/s41598-019-48994-5
16. Rathore S.K., Dwibedi B., Pati S.S., Panda S., Panda M., Sabat J., Kar S.K. An Investigation on the Coinfection Measles and HSV-1 in Hospitalized Acute Encephalitis Syndrome Patients in Eastern India. Neurol. India 2019; 67(5): 1358–9. doi: 10.4103/0028-3886.271247
17. Anaedobe C.G., Ajani T.A. Co Simplex Virus Type 2 and HIV Infections among Pregnant Women in Ibadan, Nigeria. J. Glob. Infect. Dis. 2019; 11(1): 19–24. doi: 10.4103/jgid.jgid_56_18
18. Ferreira A.C., Romão T.T., Macedo Y.S., Pupe C., Nascimento O.J.M.; Fellow of the American Academy of Neurology (FAAN). COVID-19 and Herpes zoster co-infection presenting with trigeminalneuropathy.Eur J Neurol.2020;27(9):1748-1750. doi: 10.1111/ene.14361.
19. Tartari F., Spadotto A., Zengarini C., Zanoni R., Guglielmo A., Adorno A. et al. Herpes zoster in COVID-19-positive patients. In. J. Dermatol. 2020; 59(8): 1028–9. doi: 10.1111/ijd.15001
20. Elsaie M.L., Youssef E.A., Nada H.A.. Herpes zoster might be an indicator for latent COVID-19 infection. Dermatol. Ther. 2020; 33(4): e13666. doi: 10.1111/dth.13666
21. Le Balc’h P., Pinceaux K., Pronier C., Seguin P., Tadié J.M., Reizine F. Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Crit. Care 2020; 24(1): 530. doi: 10.1186/s13054-020-03252-3
22. Sinadinos A., Shelswell J. Oral ulceration and blistering in patients with COVID-19. Evid. Based. Dent. 2020; 21(2): 49. doi: 10.1038/s41432-020-0100-z
23. Bond P. Ethnicity and the relationship between covid-19 and the herpes simplex viruses. Med. Hypotheses 2021; 146: 110447. doi: 10.1016/j.mehy.2020.110447
24. Amorim Dos Santos J., Normando A.G.C., Carvalho da Silva R.L., De Paula R.M., Cembranel A.C. et al. Oral mucosal lesions in a COVID-19 patient: New signs or secondary manifestations? Int. J. Infect. Dis. 2020; 97: 326–8. doi: 10.1016/j.ijid.2020.06.012
25. Xu R., Zhou Y., Cai L., Wang L., Han J., Yang X. et al. Co-reactivation of the human herpesvirus alpha subfamily (herpes simplex virus-1 and varicella zoster virus) in a critically ill patient with COVID-19. Br. J. Dermatol. 2020; 183(6): 1145–7. doi: 10.1111/bjd.19484
26. Cao X., Zhang X., Meng W., Zheng H. Herpes Zoster and Postherpetic Neuralgia in an Elderly Patient with Critical COVID-19: A Case Report. J. Pain. Res. 2020; 13: 2361–5. doi: 10.2147/JPR.S274199
27. Dursun R., Temiz S.A. The clinics of HHV-6 infection in COVID-19 pandemic: Pityriasisrosea and Kawasaki disease. Dermatol. Ther. 2020; 33(4): e13730. doi: 10.1111/dth.13730
28. Brambilla L., Maronese C.A., Tourlaki A., Veraldi S. Herpes zoster following COVID-19: a report of three cases. Eur. J. Dermatol. 2020; 30(6): 754–6. doi: 10.1684/ejd.2020.3924
29. Kadambari S., Klenerman P., Pollard A.J. Why the elderly appear to be more severely affected by COVID-19: The potential role of immunosenescence and CMV. Rev. Med. Virol. 2020; 30(5): e2144. doi: 10.1002/rmv.2144
30. Amaral P.H., Ferreira B.M., Roll S., Neves P.D., Pivetta L,G,, Mohrbacher S. et al. COVID-19 and Cytomegalovirus Co-infection: A Challenging Case of a Critically Ill Patient with Gastrointestinal Symptoms. Eur. J. Case Rep. Intern. Med. 2020; 7(10): 001911. doi: 10.12890/2020_001911
31. García-Martínez F.J., Moreno-Artero E., Jahnke S. SARS-CoV-2 and EBV. Med. Clin. (Engl). 2020; 155(7): 319–20. doi: 10.1016/j.medcle.2020.06.010
32. Соломай Т.В., Филатов Н.Н. Сезонность инфекции, вызванной вирусом Эпштейна–Барр. Журнал инфектологии 2020; 12(4): 93–100. doi: 10.22625/2072-6732-2020-12-4-93-100
Solomay T.V., Filatov N.N. [Seasonality of infection caused by the Epstein–Barr virus]. Zhurnal infektologii 2020; 12(4): 93–100. (In Russ.). doi: 10.22625/2072-6732-2020-12-4-93-100
33. Huang W., Berube J., McNamara M., Saksena S., Hartman M., Arshad T. et al. Lymphocyte subset counts in COVID-19 Patients: a meta-analysis. Cytometrya 2020; 97(8): 772–6. doi: 10.1002/cyto.a.24172
34. Mehta S.K., Bloom D.C., Plante I., Stowe R., Feiveson A.H., Renner A. et al. Reactivation of Latent Epstein-Barr Virus: A Comparison after Exposure to Gamma, Proton, Carbon, and Iron Radiation. Int. J. Mol. Sci. 2018;b19(10): pii: E2961. doi: 10.3390/ijms19102961
35. Баженова Л.Г., Ботвиньева И.А., Ренге Л.В., Полукаров А.Н. Динамика распространенности TORCH-инфекций у беременных. Оценка риска первичного инфицирования плода. Мать и дитя в Кузбассе 2012; (1): 22–6.
Bazhenova L.G., Botvinyeva I.A., Renge L.V., Polukarov A.N. [Dynamics of the prevalence of TORCH infections in pregnant women. Assessment of the risk of primary infection of the fetus]. Mat’ i ditya v Kuzbasse 2012; (1): 22–6. (In Russ.).
36. Островская О.В., Власова М.А., Наговицына Е.Б., Морозова О.И., Ивахнишина Н.М. Распространенность TORCH-инфекций у женщин Приамурья. Бюллетень физиологии и патологии дыхания 2008; 30: 72–7.
Ostrovskaya O.V., Vlasova M.A., Nagovitsyna E.B., Morozova O.I., Ivakhnishina N.M. [Prevalence of TORCH infections in women of the Amur Region]. Bulleten’ fiziologii i patologii dyhaniya 2008; 30: 72–7. (In Russ.).
37. Herrera-Ortiz A., Conde-Glez C.J., Vergara-Ortega D.N., García-Cisneros Olamendi-Portugal M.L., Sánchez-Alemán M.A. Avidity of antibodies against HSV-2 and risk to neonatal transmission among Mexican pregnant women. Infect. Dis. Obstet. Gynecol. 2013; 140–2. doi: 10.1155/2013/140142
38. Reward E.E., Muo S.O., Orabueze I.N.A., Ike A.C. Seroprevalence of herpes simplex virus types 1 and 2 in Nigeria: a systematic review and meta-analyses. Pathog. Glob. Health 2019; 113(5): 229–37. doi: 10.1080/20477724.2019.1678938
39. Dargham S.R., Nasrallah G.K., Al-Absi E.S., Mohammed L.I., Al-Disi R.S., Nofal M.Y., Abu-Raddad L.J. Herpes Simplex Virus Type 2 Seroprevalence Among Different National Populations of Middle East and North African Men. Sex. Transm. Dis. 2018; 45(7): 482–87. doi: 10.1097/OLQ.0000000000000791
40. Beader N., Kolarić B., Slačanac D., Tabain I., Vilibić-Čavlek T. Seroepidemiological Study of Epstein–Barr virus in Different Population Groups in Croatia. Isr. Med. Assoc. J. 2018; 20 (2): 86–90.
41. Fourcade G., Germi R., Guerber F., Lupo J., Baccard M., Seigneurin A. et al. Evolution of EBV seroprevalence and primary infection age in a French hospital and a city laboratory network, 2000–2016. PLoS One 2017; 12(4): e0175574. doi: 10.1371/journal.pone.0175574
42. Lachmann R., Loenenbach A., Waterboer T., Brenner N., Pawlita M., Michel A. et al. Cytomegalovirus (CMV) in the adult population of Germany. PLoS One 2018; 13(7): e0200267. doi: 10.1371/journal.pone.0200267
43. Cannon M.J., Schmid D.S., Hyde T.B.. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med .Virol. 2010; 20(4): 202–13. doi: 10.1002/rmv.655
44. Aimola G., Beythien G., Aswad A., Kaufer B.B. Current understanding of herpesvirus 6 (HHV-6) chromosomal integration. Antiviral Res. 2020; 176: 104720. doi: 10.1016/j.antiviral.2020.104720
45. Drago F., Ciccarese G., Rebora A., Parodi A. Human herpesvirus-6, -7, and Epstein–Barr virus reactivation in pityriasisrosea during COVID-19. J. Med. Virol. 2021; 93(4): 1850–1. doi: 10.1002/jmv.26549
46. Paolucci S., Cassaniti I., Novazzi F., Fiorina L., Piralla A., Comolli G. et al. DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. Int. J. Infect. Dis. 2020; 104: 315–9. doi: 10.1016/j.ijid.2020.12.051
47. Ablashi D., Agut H., Alvarez-Lafuente R., Clark D.A., Dewhurst S., DiLuca D., Flamand L., Frenkel N., Gallo R., Gompels U.A. Classification of HHV-6A and HHV-6B as distinct viruses. Arch. Virol. 2014; 159(5): 863–70. DOI: 10.1007/s00705-013-1902-5
48. Мелехина Е.В., Лысенкова М.Ю., Свитич О.А., Музыка А.Д., Каражас Н.В., Рыбалкина Т.Н. и др. Особенности течения инфекции ВГЧ-6А и ВГЧ-6В у детей, проживающих в Московском регионе. Эпидемиол. инфекц. болезни. Актуал. вопр. 2018; (2): 42–9. DOI: 10.18565/epidem.2018.2.42-9
Melekhina E.V., Lysenkova M.Yu., Svitich O.A., Muzyka A.D., Karazhas N.V., Rybalkina T.N. et al. [Features of HHV-6A and HHV-6B infections in children living in the Moscow Region]. Èpidemiologiâ i infekcionnye bolezni. Аktual’nye voprosy 2018; (2): 42–9 (In Russ.). DOI: 10.18565/epidem.2018.2.42-9
About the Autors
Tatiana V. Solomay, Сand. Med. Sci., Deputy Head, Interregional Department №. 1 of the FMBA of Russia; Senior Researcher, Laboratory and Epidemiological Analysis and Monitoring of Infectious Diseases, I.I. Mechnikov Research Institute of Vaccines and Serums, Moscow, Russia; solomay@rambler.ru; https://orcid.org/0000-0002-7040-7653
Professor Tatiana A. Semenenko, MD, Head, Department of Epidemiology, Honorary Academician N.F. Gamaleya National Research Center for Epidemiology and Microbiology, Ministry of Health of Russia; Professor, Department of Infectology and Virology, I. M. Sechenov First Moscow State Medical University, Ministry of Health of the Russia (Sechenov University), Moscow, Russia; semenenko@gamaleya.org; https://orcid.org/0000-0002-6686-9011
Elena I. Isayeva, Cand. Biol. Sci., Leading Researcher, Laboratory of Immunology, D.I. Ivanovsky Research Institute of Virology, Honorary Academician N.F. Gamaleya National Research Center for Epidemiology and Microbiology, Ministry of Health of Russia, Moscow, Russia; immunol.lab@mail.ru; https://orcid.org/0000-0002-2523-0692
Elizaveta N. Vetrova, Researcher, Laboratory of Immunology, D. I. Ivanovsky Research Institute of Virology, Honorary Academician N.F. Gamaleya National Research Center for Epidemiology and Microbiology, Ministry of Health of Russia, Moscow, Russia; immunol.lab@mail.ru; https://orcid.org/0000-0003-1902-5278
Alyona I. Chernyshova, Junior Researcher, Laboratory of Immunology, D. I. Ivanovsky Research Institute of Virology, Honorary Academician N.F. Gamaleya National Research Center for Epidemiology and Microbiology, Ministry of Health of Russia, Moscow, Russia; immunol.lab@mail.ru; https://orcid.org/0000-0003-1290-4042
Elina V., Romenskaya, Epidemiologist, Branch № 8, N.N. Burdenko Main Military Clinical Hospital, Ministry of Defense of the Russia, Moscow Region, Khimki, Russia; elinaromenskaya@yandex.ru; https://orcid.org/0000-0001-8097-9412
Professor Nataliya V. Karazhas, BD, Head, Laboratory of Epidemiology of Opportunistic Infections, Honorary Academician N.F. Gamaleya National Research Center for Epidemiology and Microbiology, Ministry of Health of Russia, Moscow, Russia; karazhas@inbox.ru; https://orcid. org / 0000-0003-3840-963X
Similar Articles

 Внутригодовая динамика заболеваемости инфекционным мононуклеозом характеризовалась сезонным подъемом в холодный период года и относительным эпидемическим благополучием в летние месяцы, которое в 2019 г. продолжалось с мая по август, а в 2020 г. после минимального показателя в мае (0,4 на 100 тыс. населения) сразу же был отмечен рост. Изменение заболеваемости ЦМВИ по месяцам в 2020 г. существенно не отличалось от такового в 2019 г. Как в 2019, так и в 2020 гг. выраженный сезонный подъем показателей не выявлен.
Внутригодовая динамика заболеваемости инфекционным мононуклеозом характеризовалась сезонным подъемом в холодный период года и относительным эпидемическим благополучием в летние месяцы, которое в 2019 г. продолжалось с мая по август, а в 2020 г. после минимального показателя в мае (0,4 на 100 тыс. населения) сразу же был отмечен рост. Изменение заболеваемости ЦМВИ по месяцам в 2020 г. существенно не отличалось от такового в 2019 г. Как в 2019, так и в 2020 гг. выраженный сезонный подъем показателей не выявлен.



