Острые респираторные вирусные инфекции (ОРВИ) множественной и неуточненной этиологии ежегодно составляют около 90% всей инфекционной патологии, прочно занимая первое рейтинговое место по экономическому ущербу. В 2014 г. инфекциями данной группы переболело 20% населения Российской Федерации. Показатель заболеваемости в целом по стране составил 19 505,99 на 100 тыс. населения, а экономический ущерб оценивается в 376 632 162 200 руб. – это 80,4% потерь, связанных со всеми инфекционными болезнями [1].
Среди респираторных вирусов наиболее агрессивными и контагиозными являются вирусы гриппа. Благодаря целенаправленной федеральной программе иммунопрофилактики отмечается ежегодное снижение напряженности эпидемий гриппа, особенно в детских, воинских и других организованных коллективах. Заболевшие гриппом переносят инфекцию легче и имеют меньше осложнений. Однако появление новых штаммов вируса гриппа всегда чрезвычайно опасно ввиду быстрого и беспрепятственного их распространения, что было продемонстрировано в 2009 г. на примере вируса А/ Н1N1Калифорния/04/2009. После введения в состав вакцин этого штамма вируса грипп понизил свою рейтинговую оценку экономического ущерба со 2-го места в 2009 г. до 18-го в 2014 г. [1]. В 2014 г. в целом по РФ интенсивность эпидемического процесса гриппа была невысокой, заболеваемость составила 9,04 на 100 тыс. населения, что привело к экономическим потерям в 293 939 700 руб. Самые высокие показатели (83,26 на 100 тыс. населения) были зарегистрированы в Магаданской области, а стабильно низкие – в Ростовской, Саратовской, Самарской областях и Приморском крае [1]. Центрами гигиены и эпидемиологии в субъектах Российской Федерации грипп был подтвержден лабораторными методами в 10,5% образцов клинического материала от больных гриппоподобными заболеваниями. Как и в предыдущем эпидемическом сезоне, большинство (57%) возбудителей относилось к серотипу вируса гриппа A/H3N2 (в текущем эпидемическом сезоне он не соответствовал рекомендованному ВОЗ вакцинному штамму), 24% составили вирусы гриппа А/ Н1N1Калифорния/04/2009, 16% – вирусы гриппа B, 3% – нетипируемые вирусы гриппа A (оперативные данные Роспотребнадзора с 1.12.2014 по 20.03 2015).
Основной причиной умеренной интенсивности эпидемического процесса гриппа в последние годы является увеличение охвата населения профилактическими прививками. В 2014 г. в РФ против этой инфекции привито 42,37 млн человек (в 2012 г. – 37,74 млн, в 2013 г. – 39,71 млн), что составило 29,6% населения страны [1]. Более трети от числа привитых составили дети. При анализе зарегистрированных в 2014 г. летальных исходов установлено, что практически все умершие от гриппа не были охвачены специфической иммунопрофилактикой.
Высокая изменчивость вирусов гриппа привела к необходимости ежегодной смены вакцинных штаммов. Эффективность вакцинации при этом находится в прямой зависимости от совпадения прогнозируемых и фактически циркулирующих штаммов вирусов гриппа. В связи с этим при создании вакцин нового поколения учитывается необходимость не только обеспечения безопасности при высокой специфической иммуногенности и длительности сохранения защитного титра антител, но и возможности снижения восприимчивости к прочим респираторным вирусам.
В высокой степени этим требованиям отвечают виросомальные вакцины, которые имеют ряд преимуществ по сравнению с вакцинами предыдущих поколений (цельновирионными, субъединичными и сплит-вакцинами). При сравнительном изучении гриппозных вакцин ряд авторов [2, 3] отметили, что виросомальные вакцины индуцируют более высокую иммуногенность и более длительное сохранение протективных антител по сравнению с традиционными субъединичными и расщепленными вакцинами, поэтому виросомы рассматриваются как адъювант, усиливающий иммунную реакцию организма.
В ходе проведения клинических исследований на добровольцах из различных возрастных групп австрийскими учеными было показано, что мембранный белок вируса гриппа индуцирует клеточный иммунитет и увеличивает эффективность вакцинации против гриппа [4].
В России виросомальная вакцина «Ультрикс®» была зарегистрирована в 2008 г. (регистрационное удостоверение ЛСР-001419/08), после чего прошла ряд успешных крупномасштабных полевых и клинических (III–IV фазы) исследований [5–10]. Результаты исследования иммуногенности, эффективности и безопасности применения вакцины «Ультрикс®» в эпидемический сезон 2014–2015 гг. представлены в настоящей статье.
Цель исследования – изучение иммуногенности, эффективности и переносимости вакцины «Ультрикс®» среди контингентов повышенного риска заболевания гриппом и ОРВИ (дети и обслуживающий персонал закрытого учреждения неврологического профиля, медицинские работники, преподаватели вузов, воинские коллективы) и в полевых испытаниях.
 Задачи исследования:
Задачи исследования:
- оценить иммуногенную активность вакцины «Ультрикс®» и длительность сохранения защитных антител у детей с хронической неврологической патологией и взрослых из группы повышенного риска заболевания гриппом и ОРВИ;
- изучить эффективность вакцины «Ультрикс®» для профилактики сезонного гриппа и прочих ОРВИ в сравнительном эпидемиологическом наблюдении;
- сравнить течение ОРВИ и гриппа (выраженность и длительность клинических проявлений, тяжесть заболевания, количество осложнений) в группах привитых вакциной «Ультрикс®» и другими отечественными и импортными вакцинами, а также не получивших специфической иммунопрофилактики гриппа;
- изучить переносимость вакцины (регистрация местных, общих реакций, серьезные нежелательные явления) у всего контингента привитых (полевые испытания).
Вакцина гриппозная инактивированная расщепленная «Ультрикс®» представляет собой смесь высокоочищенных протективных поверхностных и внутренних антигенов вирусов гриппа типа А(H1N1 и H3N2) и В.
В соответствии с рекомендациями ВОЗ, в 1 дозе (0,5 мл) вакцины содержатся вирусы гриппа, культивированные на куриных эмбрионах, инактивированные, расщепленные, представленные штаммами, эквивалентными следующим: А(H1N1) – 15 ± 2,2 мкг ГА; А(H3N2) – 15 ± 2,2 мкг ГА; В – 15 ± 2,2 мкг ГА. Штаммовый состав вакцины ежегодно пересматривается. Он соответствует рекомендациям ВОЗ для Северного полушария и решению Комиссии по гриппозным вакцинным и диагностическим штаммам Минздрава России.
Технология производства гриппозной вакцины «Ультрикс®» основана на новом подходе к разрушению вирионов вируса гриппа с последующей самосборкой виросом. В процессе расщепления вирионов под действием высокоэффективного детергента β-октилгликозида стало возможно максимально перевести в растворимое состояние поверхностные антигены вируса гриппа (гемагглютинин и нейраминидазу), внутренние антигены (мембранный белок и рибонуклеокапсид) в виде мицелл с сохранением их антигенной активности, а также значительную часть липидов вирусной мембраны. Благодаря применению оригинальной технологии сборки вирусных антигенов в составе вакцины липиды в сочетании с поверхностными антигенами образуют псевдовирусные структуры – виросомы. Противогриппозная вакцина «Ультрикс®» активирует гуморальный и клеточный иммунитет. Известно, что клеточный иммунитет является более кросс-реактивным для дрейфующих эпидемических штаммов вируса гриппа, а также является одним из существенных факторов защиты людей от заболевания гриппом. Способность индуцировать клеточный иммунитет может быть одним из важных факторов увеличения профилактической эффективности вакцины «Ультрикс®».
Вакцина гриппозная инактивированная расщепленная «Ультрикс®» прошла все этапы доклинических и клинических исследований, которые показали, что она безопасна, хорошо переносится привитыми, обладает низкой реактогенностью при иммунизации населения в возрасте от 6 до 60 лет и высокой иммуногенностью.
Материалы и методы
В эпидемиологическом наблюдении для анализа использовали специально разработанные листы наблюдений за вакцинированными и лицами из контрольных групп. В лист наблюдения входили персональные сведения, а также данные медицинского наблюдения (активного и пассивного) на протяжении 180–200 дней от даты вакцинации с фиксацией тяжести и длительности клинических симптомов гриппоподобных заболеваний, данных этиотропной диагностики. Всего было привито 3756 человек, в том числе 581 ребенок в возрасте от 6 до 12 лет. 2102 привитых приняли участие в открытом проспективном сравнительном эпидемиологическом наблюдении, а 1654 – в полевых исследованиях (табл. 1).
Изучение и оценку проявлений эпидемического процесса гриппа и ОРВИ проводили по данным еженедельного мониторинга.
Иммуногенность вакцины оценивали по критериям МУ 3.3.2.1758-03 2003 и СРМР ЕМЕА, СРМР/ЕWР/1045/01 [10, 11]. Использовали реакцию торможения гемагглютинации для исследования 71 парной сыворотки привитых детей (26 человек) и взрослых из группы риска по заболеванию гриппом и ОРВИ, взятых в день иммунизации и спустя 27, 95 и 201 день после иммунизации. Оценку антигенной активности вакцин проводили по числу диагностических приростов титров антител в 4 и более раз, динамике величин средних геометрических титров антител (СГТ).
Результаты
Уровень сероконверсий у привитых детей и взрослых из групп повышенного риска по заболеванию гриппом и ОРВИ с титром антител в первой сыворотке < 1:10–1:320 к вирусам А(Н1N1) pdm09, А(Н3N2) и В составил соответственно 60,5, 61,9 и 33,8%; с титром антител в первой сыворотке ≤ 1:20 соответственно 77,7, 95,8 и 40,0%. Полученные результаты подтвердили данные других исследований [5, 7, 9 ] о том, что для вирусов гриппа А (H1N1 pdm09 и H3N2) наблюдалось увеличение титров антител, значительно превышающее критерии CPMP [11], а для вирусов гриппа В процент 4-кратного прироста титра антител находился на рекомендованном CPMP уровне (для первично серонегативных испытуемых) и был чуть ниже для пациентов с титром антител в первой сыворотке < 1:10–1:320. Эти данные свидетельствует о высокой иммуногенной активности вакцины «Ультрикс®».
Кратность прироста СГТ антител в сыворотках крови пациентов с исходным титром < 1:10–1:320 для вирусов гриппа A(H1N1) pdm09, A(H3N2) и В составила 3,92, 2,89 и 2,05 соответственно, а с исходным титром ≤ 1:20 – 7,56, 7,48 и 2,51 соответственно, что также соответствует критерию СРМР. Таким образом, кратность прироста антител у серонегативных пациентов (исходный титр антител ≤ 1:20) был в 2–3 раза выше к вирусам гриппа А и в 1,2 раза выше к вирусам гриппа В, чем у пациентов с исходным титром антител < 1:10–1:320.
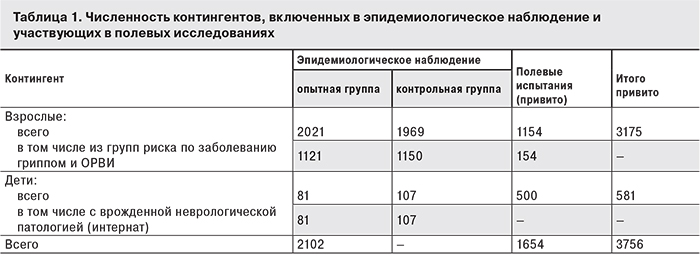
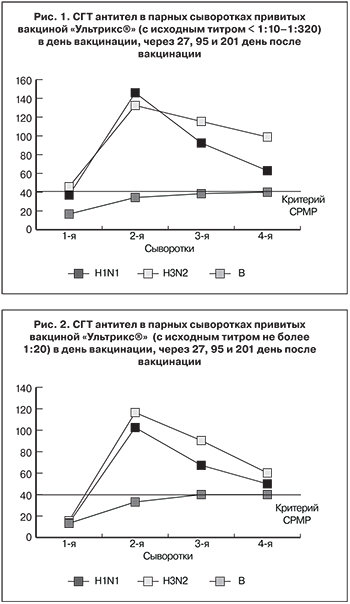
Была изучена длительность сохранения антител в сыворотках иммунизированных детей и взрослых с высоким риском заболевания гриппом и ОРВИ через 95 и 201 день после вакцинации. Сравнительные данные СГТ антител в парных сыворотках крови привитых вакциной «Ультрикс®» представлены на рис. 1 и 2.
Из данных, представленных на рис. 1 следует, что максимальный прирост антител у привитых с исходным титром < 1:10–1:320 к вакцинным штаммам вируса гриппа А выявлен через 27 дней после вакцинации, далее следовало снижение СГТ, но даже на 201-й день после вакцинации он был в 2 раза выше исходного уровня. Прирост антител к вирусам гриппа В был поступательным и к 201-му дню также превысил исходный уровень в 2 раза.
Анализируя полученные в ходе исследования данные (см. рис. 2), можно сделать вывод, что максимальный прирост антител у привитых с исходным титром не более 1:20 к вакцинным штамма вируса гриппа А выявлен через 27 дней после вакцинации; далее следовало снижение СГТ, но даже на 201-й день после вакцинации он был в 5–6 раз выше, чем исходный уровень. Прирост антител к вирусам гриппа В был поступательным и к 201-му дню превысил исходный уровень в 3 раза.
Результаты полевых испытаний
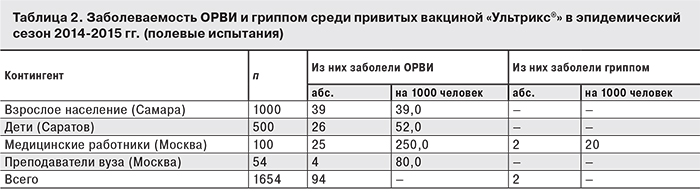
Медицинским наблюдением были охвачены 1654 привитых, в том числе 500 детей, которые проживают на 4 административных территориях (Москва, Московская область, Саратов, Самара). Результаты представлены в табл. 2.
При анализе результатов наблюдения (данные листов наблюдений и первичной медицинской документации) установлено, что случаи заболевания гриппом у привитых вакциной «Ультрикс®» выявлены только среди медицинских работников. В этой когорте зарегистрирована также самая высокая заболеваемость ОРВИ другой этиологии. Показатель заболеваемости среди медицинских работников был в 7,8 раз выше, чем среди взрослого населения Самары, в 5 раз выше, чем среди детей Саратова и в 3 раза выше, чем среди преподавателей столичного вуза.
На территории Саратова интенсивность эпидемического процесса была невысокой, в этиологической структуре доминировал вирус гриппа В: по данным вирусологического мониторинга, его доля составила 60% от числа выделенных вирусов гриппа за период с 42-й недели 2014 г. по 16-ю неделю 2015 г. Среди 500 детей, привитых вакциной «Ультрикс®», заболевших гриппом не выявлено.
Средний возраст 1000 привитых вакциной «Ультрикс®» в Самаре составил 42,2 ± 0,6 года. ОРВИ заболели 39 человек, у 26 из них отобран клинический материал (назальные смывы) для расшифровки этиологии заболеваний. Методом ПЦР выявлены РС-вирусы, аденовирусы, риновирусы, вирусы парагриппа. По данным вирусологического мониторинга по Самарской области, за анализируемый период среди 64 лабораторно подтвержденных случаев гриппа 2 (вызваны вирусами гриппа А/H3N2 и В) зарегистрированы у привитых другими противогриппозными вакцинами.
Результаты многоцентрового открытого сравнительного эпидемиологического наблюдения
В октябре–ноябре 2014 г. в поликлинике ФКУЗ «МСЧ МВД России по Белгородской области» были иммунизированы вакциной Ультрикс® 900 человек, они составили опытную группу. В контрольную группу вошли 900 человек, которые не были привиты против гриппа и не получали средств неспецифической профилактики гриппа. Сравнительные результаты заболеваемости ОРВИ в опытной и контрольной группах представлены в табл. 3.
Уровень заболеваемости ОРВИ в опытной группе был в 2,4 раза ниже, чем в контрольной. Обращает на себя внимание также преобладание у заболевших в опытной группе легкого течения ОРВИ, отсутствие осложненных форм заболевания.
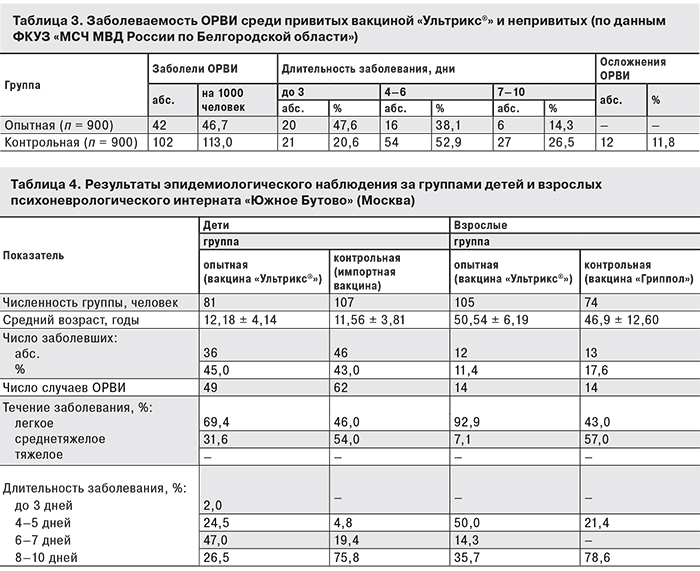
В психоневрологическом интернате «Южное Бутово» (Москва) вакциной «Ультрикс®» были привиты 81 ребенок и 105 человек медицинского персонала (опытные группы). В контрольные группы вошли соответственно дети и взрослые того же учреждения, привитые другими вакцинами (импортной и отечественной). Результаты эпидемиологического наблюдения представлены в табл. 4.
Случаев заболевания гриппом в интернате среди лиц, находящихся под наблюдением, не выявлено. Методом ПЦР из клинического материала от детей и сотрудников с гриппоподобными заболеваниями из опытной группы выделены вирусы парагриппа и аденовирусы.
Данные табл. 4 позволяют сделать вывод о том, что противогриппозные вакцины снижают восприимчивость ослабленных основным заболеванием детей и взрослых, работающих в закрытых детских коллективах, к разным респираторным вирусам. При этом 69,4% заболевших ОРВИ взрослых и 92,9% заболевших детей из опытных групп переносили инфекцию преимущественно в легкой форме со средней продолжительностью заболевания 5,12 ± 1,4 и 5,87 ± 2,1 дня соответственно. Заболевшие взрослые и дети контрольных групп, привитые другими вакцинами, переносили ОРВИ в легкой форме в 46,0 и 43,0% случаев при средней продолжительности заболеваний 6,6 ± 1,75 и 6,78,1 ± 2,16 дня соответственно.
Эпидемиологическую эффективность гриппозной инактивированной расщепленной вакцины «Ультрикс®» для снижения уровня заболеваемости гриппом и ОРВИ оценивали в организованных коллективах военнослужащих в период их формирования. В основную группу вошли 1016 военнослужащих, привитых вакциной «Ультрикс®» с 14 по 28 декабря 2014 г., в период формирования нового воинского коллектива. Контрольную группу составили 995 невакцинированных военнослужащих, имеющих аналогичные условия размещения, питания, труда, отдыха и военно-профессиональной деятельности. Средний возраст испытуемых составил 19,3 ± 1,6 года.
Учитывали и анализировали случаи заболевания военнослужащих гриппом и ОРВИ. Данные представлены на рис. 3.
Исходный уровень заболеваемости ОРВИ и гриппом в основной и контрольной группах в декабре 2014 г. статистически достоверно не различался: ОРВИ – 306,3 и 367,9‰ (χ2 = 0,67; p > 0,05), грипп – 9,0 и 9,4‰ (χ2 = 0,45; p > 0,05).
В январе–феврале 2015 г. зарегистрирован эпидемический подъем заболеваемости ОРВИ и гриппом. Данные оперативного эпидемиологического анализа свидетельствовали о том, что заболеваемость ОРВИ среди военнослужащих основной группы достоверно снизилась с 306,3‰ в декабре до 171,2‰ в феврале (χ2 = 4,86; p < 0,05), в то время как в контрольной группе она увеличилась в 1,4 раза – с 367,9‰ в декабре до 518,9‰ в феврале (χ2 = 4,30; p < 0,05). Уровень заболеваемости ОРВИ в феврале 2015 г. в основной группе был в 3,0 раза ниже, чем в контрольной – 171,2 и 518,9‰ соответственно (χ2 = 27,64; p < 0,05).
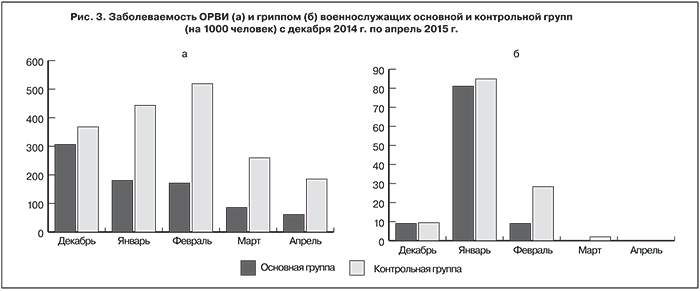
Достоверных отличий в уровнях заболеваемости гриппом в январе 2015 г. в основной и контрольной группах не выявлено (p > 0,05). Необходимо отметить, что заболеваемость в обеих группах увеличилась в 9 раз. Она была вызвана вирусом гриппа А/ H3N2, имеющим иную антигенную структуру, чем вакцинный штамм.
Изучение реактогенности вакцины «Ультрикс®»
В ходе наблюдения за 3756 привитыми вакциной «Ультрикс®» в течение первых 20 мин. после вакцинации и далее в течение 48 ч выявлено 39 человек (1,04%) с местными реакциями в области введения вакцины, которые прошли в пределах двух суток. Ни у одного из вакцинированных не выявлено серьезных нежелательных явлений.
Чрезвычайно важен тот факт, что у 81 привитого ребенка с тяжелой неврологической патологией не выявлено местных и общих реакций на введение вакцины «Ультрикс®», что еще раз подтверждает ее ареактогенность и хорошую переносимость.
Выводы
1. Установлена высокая иммуногенная активность вакцины «Ультрикс®». Уровень сероконверсии у привитых с высокими рисками заболевания гриппом и ОРВИ с титром антител в первой сыворотке < 1:10–1:320 к вирусам А(Н1N1) pdm09, А(Н3N2) и В составил соответственно 60,5, 61,9, 33,8%; с титром антител в первой сыворотке ≤ 1:20 соответственно 77,7, 95,8 и 40,0%. Полученные показатели значительно выше критерия СРМР для вакцинных штаммов вирусов гриппа А и находится на уровне требуемого критерия для вируса гриппа В.
2. Доказано сохранение протективного уровня антител ко всем трем вакцинным штаммам вирусов гриппа не менее 201 дня после вакцинации. Максимальный прирост СГТ антител к вакцинным штаммам вируса гриппа А выявлен через 27 дней после вакцинации; на 201-й день после вакцинации у первично серонегативных испытуемых он был соответственно в 5–6 (для вируса гриппа А) и 2–3 раза (для вируса гриппа В) выше исходного уровня.
3. В многоцентровом сравнительном эпидемиологическом наблюдении показана эффективность вакцины «Ультрикс®» в отношении вирусов гриппа и других ОРВИ. Применение вакцины приводит к достоверному уменьщению заболеваемости ОРВИ, снижению тяжести и длительности течения заболеваний, предотвращает тяжелые осложнения.
4. Вакцина «Ультрикс®» является ареактогенным профилактическим препаратом. Число местных реакций у 3756 привитых составило 1,04%, что соответствует требованиям к вакцинным препаратам.
5. Полученные результаты дают основания рекомендовать вакцину «Ультрикс®» для ежегодной профилактической вакцинации против гриппа детей и взрослых, в том числе организованных воинских коллективов в период их формирования в осенне-зимнее время.


