Грипп остается важной причиной заболеваемости и смертности во всем мире. Сегодня, по оценкам ВОЗ, каждый год 5–10% взрослых в мире страдают от гриппа. 3–5 млн случаев заболевания проходят в тяжелой форме, в результате чего ежегодно умирают 250 000–500 000 человек [1]. В последние 40 лет в человеческой популяции циркулирует одновременно несколько серотипов вируса гриппа: A(H1N1) (до 2009 г.), A(H3N2) и вирус гриппа В двух генетических линий – В/Виктория и В/Ямагата. В 2009 г. появился новый штамм пандемического вируса гриппа H1N1. С апреля 2009 г. по август 2010 г. зарегистрировано 18 500 смертельных случаев от лабораторно подтвержденного пандемического вируса гриппа (H1N1pdm2009) [2]. Известно, что тяжелее всего заболевание протекает у иммунокомпрометированных лиц: детей в возрасте от 6 мес. до 3 лет, беременных и пожилых [1].
В настоящее время для профилактики гриппа используют аттенуированные (живые) и инактивированные вакцины, приготовленные на основе эпидемически актуальных штаммов вирусов гриппа А и В. Поскольку вирус гриппа отличается высокой изменчивостью, штаммы, на основе которых готовят сезонные вакцины, приходится менять каждые 2–3 года, а вакцинацию проводить не реже 1 раза в 2 года. Однако пациенты с иммунодефицитом отвечают на вакцинацию слабее, чем люди без иммунных отклонений, и для них показана ежегодная вакцинация.
Целью нашего исследования стало изучение влияния ВИЧ-инфекции на степень тяжести гриппа и прогноз у детей и взрослых, возможности профилактики гриппа на современном этапе.
ВИЧ-инфекция широко распространена в мире. В 2016 г. в мире число людей, живущих с ВИЧ, составляло, по разным оценкам, от 30,8 до 42,9 млн человек. В 2016 г. было зарегистрировано в среднем 1,8 (1,6–2,1) млн новых случаев заражения ВИЧ [3].
По данным M.B. Klein и соавт. грипп является одной из основных причин ОРВИ у ВИЧ-инфицированных. Так, из 50 пациентов с ВИЧ у 22 (44%) был лабораторно подтвержден грипп [4]. Схожие данные получили S. Garg и соавт., проанализировав скорость сероконверсии гриппа A (H1N1pdm09) (увеличение титра антител в 4 раза в реакции гемагглютинации) у ВИЧ-инфицированных гомосексуалистов в Тайланде через 1 и 2 года после начала распространения нового штамма вируса гриппа. Сероконверсию гриппа A (H1N1pdm09) у ВИЧ-инфицированных авторы обнаружили у 55 (35%) из 2157 обследованных [5]. В Южной Африке в сезоны 2009–2011 гг. при поражении нижних дыхательных путей грипп в 4–8 раз чаще выявляли у пациентов с ВИЧ-инфекцией [6].
J.C. Lin и соавт. [7] провели крупное ретроспективное исследование смертности от гриппа и пневмонии среди ВИЧ-инфицированных пациентов в США в сезоны 1991–1992, 1992–1993 и 1993–1994 гг. и обнаружили повышение уровня смертности до 19,74, 15,38 и 10,17 на 10 000 ВИЧ-инфицированных соответственно, что в 6,9–14,1 раза выше показателей смертности от пневмонии или гриппа среди взрослых и подростков старше 13 лет в общей популяции в США. В наиболее пораженной ВИЧ-инфекцией возрастной группе 25–54 лет смертность от пневмонии и гриппа ВИЧ-инфицированных была в 81 и 155 раза выше, чем в этой возрастной группе в целом. По оценкам авторов, риск смерти от гриппа составляет от 9,4 до 14,6 на 10 000 больных СПИДом по сравнению с 0,09–0,10 на 10 000 человек среди лиц в возрасте от 25 до 54 лет и от 6,4 до 7 на 10 000 человек среди лиц в возрасте 65 лет и старше. С внедрением ВААРТ ситуация в большинстве стран изменилась. K.M. Neuzil и соавт. [8] сравнили тяжесть течения гриппа у пациентов в возрасте от 15 до 50 лет, больных СПИДом, в сезоны гриппа до начала широкого применения ВААРТ (1995) и в течение 4 лет ее применения (1996–1999 гг.) и отметили снижение смертности после внедрения ВААРТ. C. Cohen и соавт. [9] ретроспективно проанализировали смертность от гриппа среди больных СПИДом в возрасте 25–54 лет в Южной Африке (1998–2005) и в США в период до применения ВААРТ (1987–1994) и в эпоху ВААРТ (1997–2005). Авторы выявили, что в США до применения ВААРТ смертность от гриппа среди этих больных была в 150–208 раз выше, чем смертность от всех причин среди населения в целом и в 2,5–4,1 раза выше, чем в группе лиц старше 65 лет. После введения ВААРТ смертность, связанная с гриппом, среди взрослых больных СПИДом снизилась в 3–6 раз, но осталась выше, чем в общей популяции. Показатели смертности, связанной с гриппом, среди взрослых южноафриканцев, больных СПИДом, в 1998–2005 гг. были аналогичны показателям в США в эпоху до ВААРТ.
В Южной Африке даже в 2009–2013 гг. при тяжелом течении гриппа смертность среди ВИЧ-инфицированных (22 из 419), в том числе не получавших АРТ (8 из 1113), была выше, чем среди пациентов без ВИЧ (13 из 620) – 5, 7 и 2% соответственно (p = 0,006) [10]. В целом в 1999–2009 гг. в Южной Африке смертность от гриппа среди ВИЧ-инфицированных, не страдающих туберкулезом, составила 27 на 100 000 человек (1125 человек), а среди не инфицированных ВИЧ без туберкулеза – всего 2 на 100 000 человек (937 человек), то есть была в 13,5 раз выше [11].
В 1998–1999 гг. M.P. Golden и соавт. [12] изучили течение гриппа и его влияние на прогрессию ВИЧ-инфекции у 12 пациентов, средний возраст которых составил 38,5 года. Из них 7 человек были вакцинированы от гриппа, но 1 заболел спустя 60 дней после вакцинации, 4 – в течение 90 дней, 1 – в течение 120 дней и 1 – в течение 150 дней. Все они получали АРТ. Развитие гриппа несмотря на вакцинацию авторы связывали с низким уровнем CD4+-лимфоцитов (в среднем 270 клеток/мкл). Ни один из пациентов не получал противовирусной терапии гриппа, им не потребовалась госпитализация в ОРИТ. Фебрильная лихорадка была у 6 из 12 пациентов, кашель – у 12, миалгии – у 10, тахикардия – у 5, головная боль – у 5 и боль в горле – у 4. В 6 случаях к гриппу присоединились бактериальные инфекции: у 2 пациентов – бактериальная пневмония, у 2 – бронхит, у 1 – средний отит, у 1 – синусит. Достоверных отличий в тяжести течения гриппа у ВИЧ-инфицированных на фоне проводимой АРТ и у пациентов без ВИЧ-инфекции авторами не установлено. Дальнейшее наблюдение в течение 5 мес. за ВИЧ-инфицированными пациентами не установило прогрессирования ВИЧ-инфекции после перенесенного грипа. Не отметили прогрессирования ВИЧ-инфекции после перенесенного гриппа и D.J. Skiest и соавт. [13]. M.B. Klein и соавт. [14] у ВИЧ-инфицированных пациентов со средним уровнем CD4+-лимфоцитов 325 клеток/мкл и вирусной нагрузкой (ВН) менее 50 копий/мл, получающих АРТ, также не обнаружили достоверных различий в тяжести течения гриппа по сравнению с неинфицированными.
Тем не менее, даже после широкого внедрения ВААРТ, частота госпитализации ВИЧ-инфицированных с гриппом по-прежнему превышает частоту госпитализации общего населения и сопоставима с показателями в других группах высокого риска. Так, A.D. Fine и соавт. [15] отмечают сходство клинических проявлений гриппа у пациентов с ВИЧ-инфекцией и без нее, но большую частоту госпитализаций по поводу гриппа среди ВИЧ-инфицированных. D. Skiest и соавт. в 2001 г. описали 43 случая гриппа у ВИЧ-инфицированных со средним количеством CD4+-лимфоцитов 340 клеток/мкл. Большинство пациентов имели типичные признаки и симптомы гриппа, включая кашель (90%), миалгию (64%) и лихорадку (52%). Боль в горле и головную боль испытывали менее половины пациентов. Никаких новых или необычных клинических проявлений не наблюдалось. Частота легочных осложнений была сходна с таковой у ВИЧ-негативных пациентов, лишь частота госпитализации была выше, чем обычно у ВИЧ-инфицированных лиц [16].
A.D. Fine и соавт. [15] изучали течение гриппа у ВИЧ-инфицированных с низким уровнем CD4+-лимфоцитов (от 25 до 394 клеток/мкл, в среднем 191 клетка/мкл). У 13% больных течение гриппа было более длительным, чем у пациентов без ВИЧ-инфекции; 8% потребовалась неотложная помощь; число пациентов с ВИЧ-инфекцией, которым потребовалась госпитализация, было в 100 раз больше числа пациентов без ВИЧ того же возраста.
H.M. Radwan и соавт. [17] описали течение подтвержденного гриппа А у 7 ВИЧ-инфицированных в сезон 1997–1998 гг. У трех из них развилась вирусная пневмония с одышкой, продуктивным кашлем, хрипами в легких, очаговыми или диффузными инфильтратами в легких, при этом бактериальная микрофлора в мокроте не была обнаружена. Ни один из этих пациентов не нуждался в ИВЛ.
C.E. Ormsby и соавт. [18] изучали течение пандемического гриппа H1N1pdm2009 у ВИЧ-инфицированных с количеством CD4+-лимфоцитов менее 100 клеток/мкл и выявили, что у них грипп протекал тяжелее, чем у ВИЧ-инфицированных пациентов, длительно получающих ВААРТ. Отмечено более продолжительное (до 20 дней) пребывание в больнице, более частое использование ИВЛ (23,3%) и гибель пациентов (20%), несмотря на терапию осельтамивиром (83,3% погибших). Авторы отмечают, что больным ВИЧ-инфекцией с количеством CD4+-лимфоцитов менее 100 клеток/мкл позже назначали осельтамивир (в 3 из 30 случаев он был назначен после 10–15-го дня госпитализации!), что достоверно повышало риск летального исхода. Этих пациентов позже госпитализировали [16 из 30 человек (53,3%) – после 10-го дня от начала болезни], так как начало гриппа было замаскировано оппортунистическими инфекциями, что также существенно влияло на исход заболевания. Все умершие пациенты были госпитализированы позднее 10-го дня от начала болезни. В ходе исследования были выявлены случаи длительной (более недели) персистенции вируса гриппа у ВИЧ-инфицированных несмотря на противовирусную терапию, что может свидетельствовать о риске формирования в будущем резистентных к осельтамивиру штаммов. P. Patel и соавт. [19] при ПЦР-исследовании обнаружили, что у ВИЧ-инфицированных со средним количеством CD4+-лимфоцитов 317 клеток/мкл и РНК ВИЧ менее 400 копий/мл, получающих ВААРТ, вирус гриппа сохраняется в носоглоточной слизи от 6 до 15 дней, несмотря на проводимую терапию.
J. López-Aldeguer и соавт. [20], изучив течение пандемического гриппа H1N1pdm2009 у 43 ВИЧ-инфицированных пациентов с количеством CD4+-лимфоцитов менее 200 клеток/мкл, выявили более тяжелое течение гриппа у ВИЧ-инфицированных с выраженным иммунодефицитом, более частое развитие у них бактериальных осложнений (пневмония зафиксирована у 51,2%, в том числе двухсторонняя – у 18,6%) и большую продолжительность госпитализации (13,2 дня при против 8,8 дней у пациентов с количеством CD4+-лимфоцитов более 200 клеток/мкл). На тяжесть течения заболевания влияло также назначение осельтамивира. У больных, начавших лечение в течение 48 ч после появления симптомов, в тяжелой форме грипп протекал в 30,8% случаев, а у начавших лечение после 48 ч – в 66,6% (p = 0,035). При этом риск летального исхода увеличивался в 4 раза.
P.J. Peters и соавт. [21] изучали течение пандемического гриппа H1N1pdm2009 у пациентов со средним количеством CD4+-лимфоцитов 233 клетки/мкл, 71% из которых получал АРТ. Тяжесть течения гриппа у этих пациентов и у пациентов без ВИЧ была сопоставима: интенсивная терапия потребовалась 29 и 34% соответственно, ИВЛ – 21 28%, умерли 13% больных в каждой группе. Единственным существенным отличием была большая частота диареи у ВИЧ-инфицированных – 42% против 20%.
По данным R. Kulkarni и соавт. [22], из 5 описанных случаев подтвержденного гриппа H1N1pdm2009 у детей, не получавших ВААРТ, в 4 случаях развились ОРДС и пневмония, тогда как А. Noguera-Julian и соавт. [23] у детей, зараженных пандемическим вирусом гриппа (H1N1pdm2009) на фоне ВААРТ, отличий по сравнению с детьми без ВИЧ-инфекции не обнаружили. Таким образом, применение ВААРТ имеет важное значение для предотвращения тяжелого течения пандемического гриппа как у взрослых, так и у детей.
Несмотря на то что на фоне ВААРТ у больных ВИЧ-инфекцией с количеством CD4+-лимфоцитов более 200 клеток/мкл и пациентов без ВИЧ степень тяжести гриппа практически не отличается, он остается серьезной проблемой у пациентов с поздними стадиями ВИЧ-инфекции, и особый интерес вызывают подходы к вакцинопрофилактике гриппа [24].
Ежегодная вакцинация – основная защита от гриппа и его осложнений [25], хотя в последнее время стали появляться данные о снижении эффективности вакцинопрофилактики гриппа при многократной вакцинации и более частом развитии заболевания у таких пациентов по сравнению с однократно вакцинированными в текущий эпидсезон [26].
В США вакцинация от гриппа ВИЧ-инфицированных рекомендуется с начала 1990-х годов. Одна из первых публикаций D.D. Ho [27] вызвала много споров, так как автором было зафиксировано увеличение РНК ВИЧ после вакцинации против гриппа. С тех пор было опубликовано несколько работ, как поддерживающих, так и опровергающих эту точку зрения. Рядом исследователей было установлено, что повышение ВН может быть кратковременным, и она возвратится к прежнему уровню в течение нескольких недель [27–32]. Однако в 2001 г. F. Gutiérrez и соавт. [33], изучив влияние вакцинации от гриппа на течение ВИЧ-инфекции у 6 пациентов, не получавших АРТ, обнаружили у них через 4 и 6 нед. значительное снижение количества CD4+-лимфоцитов – в среднем на 49,8%, а у 3 человек – более чем на 60%. S. Banic и соавт. [34] изучили эффективность вакцинации у 13 ВИЧ-инфицированных, получавших АРТ, и отметили отсутствие у них нарастания ВН после вакцинации. A.R. Zanetti и соавт. [35] исследовали эффективность вакцинации у 72 ВИЧ-инфицированных взрослых пациентов: с количеством CD4+-лимфоцитов менее 200 клеток/мкл (группа 1), от 200 и 500 клеток/мкл (группа 2) и более 500 клеток/мкл (группа 3). 63,9% больных получали АРТ. Никаких существенных изменений количества CD4+-лимфоцитов или ВН ни в одной группе отмечено не было. Несмотря на вакцинацию, гриппоподобное заболевание развилось у 18 (25%) человек, в том числе у 6 с титром антител после вакцинации 1:40 и выше и у 12 с отсутствием ответа на вакцину, но никому не потребовалась госпитализация, и ни у кого не развилась пневмония.
Таким образом, различия в размерах и особенностях выборки ВИЧ-инфицированных (факторы риска, стадии ВИЧ-инфекции, наличие или отсутствие АРТ), а также различная чувствительность лабораторных методов количественной оценки ВН можгут объяснить, по крайней мере, часть этих споров. В 2000 г. в 113 медицинских клиниках 10 городов США было проведено исследование влияния вакцинации от гриппа на прогрессирование ВИЧ-инфекции и развитие СПИДа у 36 050 ВИЧ-инфицированных лиц. Достоверных различий в скорости снижения количества CD4+-лимфоцитов и нарастании ВН, а также влиянии на сроки развития СПИДа и смерти у вакцинированных и невакцинированных не установлено [36]. S.A. Tasker и соавт. [37] в рандомизированном двойном слепом плацебо-контролируемом исследовании получили такие же результаты.
F.P. Kroon и соавт. [38] изучали влияние количества CD4+-лимфоцитов и ВААРТ на эффективность вакцинопрофилактики гриппа и установили, что при количестве CD4+-лимфоцитов менее 200 клеток/мкл (в среднем 56 клеток/мкл) без ВААРТ гуморальный ответ на вакцину отсутствовал, а при высоком уровне CD4+-лимфоцитов или при применении ВААРТ у пациентов с количеством CD4+-лимфоцитов менее 200 клеток/мкл (в среднем 85 клеток/мкл) антитела формировались.
Существенные изменения претерпели схемы вакцинации от гриппа ВИЧ-инфицированных. Первоначально применявшаяся однократная вакцинация моно- или трехвалентной вакциной продемонстрировала недостаточную эффективность [39–42], и заболевание развивалось несмотря на вакцинацию [14, 35]. В исследовании М.И. Klein и соавт. [14] частота достижения титра антител 1:40 и выше после вакцинации у пациентов со средним количеством CD4+-лимфоцитов 325 клеток/мкл, получающих ВААРТ, составляла 76%, но впоследствии у 40% вакцинированных развивался подтвержденный грипп А или В, что свидетельствует о низкой эффективности вакцины у ВИЧ-инфицированных. В исследовании M. Bickel и соавт. [39] иммуногенность однократно введенной трехвалентной вакцины у ВИЧ-инфицированных составила всего 69%. Подобные данные получили M. Rasoolinejad и соавт. [43]: уровень сероконверсии к штаммам гриппа A (H1N1), A (H3N2) и B оказался равным 58,5, 67 и 64,5% соответственно. По данным Р. Tebas и соавт. [40], даже у пациентов со средним количеством CD4+-лимфоцитов 502 клетки/ мкл, получавших ВААРТ, при однократной вакцинации моновалентной инактивированной вакциной против гриппа H1N1 2009 в дозе 15 мкг иммуногенность составила всего 60% [40]. В исследовании Y.F. Lau и соавт. [42] титр 1:40 через 3 нед. после вакцинации трехвалентной вакциной был достигнут лишь у 35% ВИЧ-инфицированных для гриппа A (H1N1, у 43% – для А H3N2 и у 19% – для гриппа B.
В 2008 г. M.J. Levin и соавт. [44] опубликовали результаты крупного исследования эффективности и безопасности различных противогриппозных вакцин. В него были включены 243 ребенка в возрасте от 5 до 18 лет, получавших АРТ по схеме ZDV + 3TC + ABC. У детей, получавших живую аттенуированную вакцину по сравнению с детьми, получавшими инактивированную трехвалентную вакцину, в 2 раза чаще развивались побочные эффекты: назофарингит – у 62 и 34% соответственно, абдоминальные симптомы – у 18 и 9% и т. д., то есть инактивированная трехвалентная вакцина для ВИЧ-инфицированных является более безопасной.
В 2009 г. были опубликованы результаты рандомизированного двойного слепого контролируемого исследования эффективности субъединичных и вирусомальных (Influvac) вакцин против гриппа для пациентов с ослабленным иммунитетом, в том числе ВИЧ-инфицированных. Установлено, что эффективность вакцинации в первом случае была выше [45].
Одним из потенциальных способов достижения более высоких показателей серопротекции при вакцинации у ВИЧ-инфицированных может быть введение бустерной дозы вакцины против гриппа [46]. До внедрения ВААРТ был опубликован ряд работ о низкой эффективности бустерной дозы вакцины. P.G. Miotti и соавт. [47] изучали эффективность вакцинации у 109 пациентов: ВИЧ-серонегативных гетеросексуальных мужчин (n = 11), ВИЧ-серонегативных гомосексуальных мужчин (n = 20), бессимптомных ВИЧ-серопозитивных мужчин (n = 32), ВИЧ-серопозитивных мужчин со СПИД-ассоциированным комплексом (ARC) (n = 9) и ВИЧ-серопозитивных мужчин со СПИДом (n = 37). Количество CD4+-лимфоцитов было определено у 67 пациентов с ВИЧ: у бессимптомных ВИЧ-серопозитивных мужчин – 527 ± 252 клетки/мкл, у пациентов с ARC – 295 ± 172 клеток/мкл и у ВИЧ-серопозитивных мужчин со СПИДом –128 ± 116 клеток/мкл. Исходные уровни РНК ВИЧ не изучали. 13 больных СПИДом были вакцинированы во время приема АРТ. Пациентам вводили 15 мкг трехвалентной субвирионной вакцины (A/Тайвань/1/86/H1N1, A/Ленинград/360/86/H3N2 и B/AnnArbor/1/86). Частота сероконверсии для каждого вакцинного антигена гриппа А варьировала от 55 до 75% после 1-й дозы и незначительно возрастала после 2-й – от 73 до 80%. Частота серологических реакций на один или оба антигена была обратно пропорциональна тяжести течения ВИЧ-инфекции. После однократной дозы вакцины пациенты с бессимптомной ВИЧ-инфекцией с титрами антител перед вакацинацией 1:16 или менее достигали сероконверсии (1:40 и выше) так же часто, как неинфицированные ВИЧ участники (ВИЧ-серонегативные гетеросексуалы и ВИЧ-серонегативные гомосексуалы): A/Тайвань/1/86/H1N1 – 80, 71 и 93% соответственно, выше A/Ленинград/360/86/H3N2 – 84, 92 и 100%, выше B/AnnArbor/1/86 – 37, 65 и 70%. Пациенты с более поздними стадиями ВИЧ-инфекции имели низкие показатели сероконверсии: A/Тайвань/1/86/H1N1 – от 38 до 67%, A/Ленинград/360/86/H3N2 – от 0 до 67% и B/AnnArbor/1/86 – от 12 до 22%. Бустерная доза практически не влияла на увеличение доли защитных антител у пациентов с бессимптомной ВИЧ-инфекцией или у пациентов со СПИДом/АRС. Введение бустерных доз вакцины минимально увеличивало долю пациентов с защитными антителами к каждому из вакцинных антигенов, и чаще это наблюдалось у здоровых пациентов из группы контроля, чем у пациентов со СПИДом/АRС (A/Тайвань/1/86/H1N1 – 90% против 55%, р = 0,07; A/Ленинград/360/86/H3N2 – 84% против 30%, p < 0,01; B/AnnArbor/1/86 – 56% против 20%, p = 0,03). Отмечено, что ответ на все антигены у пациентов со СПИДом/АRС был значительно ниже, чем у здоровых пациентов из группы контроля. Было установлено также, что титр антител после бустера коррелирует с количеством CD4+-лимфоцитов (р < 0,03). Следует отметить, что у больных СПИДом, принимавших зидовудин, двухэтапная вакцинация была эффективнее, чем у пациентов, не получавших АРТ [47]. А.М. Iorio и соавт. [48] также пришли к выводу, что бустерное дозирование противогриппозной вакцины неэффективно в группе ВИЧ-серопозитивных бывших потребителей инъекционных наркотиков. Исследование было ограничено небольшим размером выборки, отсутствием описания клинического статуса пациентов и тем фактом, что оно проводилось на популяции до введения ВААРТ.
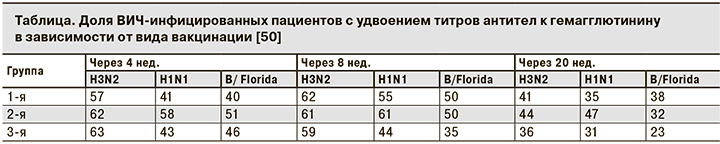
F.P. Kroon и соавт. [49] оценили эффект удвоения дозы гемагглютинина до 30 мкг в когорте ВИЧ-инфицированных пациентов и пришли к выводу, что эта стратегия неэффективна, но размер выборки был небольшим, и исследование проводили до введения ВААРТ. В дальнейшем C. Cooper и соавт. [50] в рандомизированном многоцентровом контролируемом исследовании доказали неэффективность удвоения дозы трехвалентной неоадъювантной вакцины от гриппа (A/Brisbane/59/2007/H1N1, A/Uruguay/716/2007/H3N2 и B/Флорида/4/2006). Участников исследования разделили на 3 группы. Пациенты 1-й группы были вакцинированы двукратно с интервалом 28 дней стандарноной дозой противогриппозной вакцины (15 мкг). Пациенты 2-й группы получали двойную дозу (30 мкг) двукратно с тем же интервалом. Пациенты 3-й группы получала только 1 взрослую дозу (0,5 мл или 15 мкг) вакцины. Инъекции плацебо не использовали. Среднее количество CD4+-лимфоцитов у пациентов составляло 470 клеток/мкл. Большинство ВИЧ-инфицированных получали ВААРТ. Достоверных различий между показателями пациентов 1-й и 2-й групп не выявлено (см. таблицу), но доказана большая эффективность двукратной вакцинации.
D. Soonawala и соавт. [51] изучали результаты двукратной вакцинации моновалентной MF59-адъювантной вакциной, содержащей 7,5 мкг гемагглютинина штамма A/California/7/2009/H1N1, ВИЧ-инфицированных пациентов со средним количеством CD4+-лимфоцитов 500 клеток/мкл, получавших ВААРТ. Через 21 день иммуногенность у больных ВИЧ-инфекцией составила 88%, после 2-й дозы вакцины (на 56-й день) – 91%, что было сопоставимо с результатами вакцинации пациентов без ВИЧ (89 и 93% соответственно). Дальнейшие исследования адъювантных вакцин против гриппа доказали их эффективность у ВИЧ-инфицированных и сопоставимость иммуногенности с результатами, полученными у пациентов без ВИЧ, особенно при двукратной вакцинации [52–53]. Так, по данным C. Cooper и соавт. [54], у ВИЧ-инфицированных пациентов со средним количеством CD4+-лимфоцитов 500 клеток/мкл и ВН < 50 копий/мл при вакцинации моновалентной инактивированной расщепленной адъювантной (AS03) вакциной после 1-й дозы иммуногенность составила 80%, после 2-й – 94%, а по данным A.B. Nielsen и соавт. [55] – 77,4 и 97,7% соответственно.
K. Pauksens [56], сравнив результаты двукратной вакцинации моновалентной инактивированной расщепленной адъювантной (AS03) и трехвалентной вакциной, доказал, что адъювантная вакцина эффективнее. При применении трехвалентной вакцины через 1 мес. после вакцинации иммуногенность к A/Brisbane/59/2007/H1N1 составила 53%, к A/Uruguay/10/2007/H3N2 – 63%, к B/Brisbane/60/2008 – 42%, а у получивших адъювантную вакцину – почти 70% после 1-й дозы и почти 90% – после 2-й. Однако M. Bickel и соавт. [57] у ВИЧ-инфицированных с количеством CD4+-лимфоцитов 463–504 клеток/мкл, получающих ВААРТ, после однократной вакцинации адъювантной (AS03) вакциной выявили уровень серопротекции 52,2%, а после 2-й дозы – всего 57,3%. Еще одним доводом в пользу двукратной вакцинации от гриппа ВИЧ-инфицированных является существенное снижение у них серопротекторного ответа с 54% на 28-й день после вакцинации до 28% через 6 мес. [58]. В связи с этим E.R. Glinka и соавт. [59] предложили начинать вакцинацию от гриппа непосредственно во время эпидемического подъема заболеваемости, а не в начале сезона. Среди рано вакцинированных пациентов с ВИЧ гриппом заболели 30 человек из 2773 (1,1%), среди вакцинированных поздно – 7 из 1802 (0,4%) (р = 0,01).
Т-клеточный иммунитет играет ведущую роль в борьбе с вирусом гриппа, поэтому ряд работ посвящен изучению влияния вакцинации на это звено иммунитета. C. Agrati и соавт. [60] рассмотрели влияние применения вакцины от гриппа A/California/7/2009/H1N1/ на гуморальный и клеточный иммунитет у ВИЧ-инфицированных пациентов с количеством CD4+-лимфоцитов более 200 клеток/мкл, получавших ВААРТ. Было доказано, что вакцинация позволяет эффективно индуцировать не только специфический гуморальный, но и Т-клеточный иммунитет. Полученные результаты были сходны с результатами у здоровых людей.
В последнее время изучается эффективность противогриппозной вакцины в зависимости от способа ее введения. Y.B. Seo и соавт. [61] в многоцентровом рандомизированном контролируемом открытом исследовании сравнили эффективность трех способов вакцинации – внутримышечного, интрадермального и внутрикожного – у взрослых в возрасте от 18 до 60 лет со среднем количеством CD4+-лимфоцитов 483 клеток/мкл, получающих ВААРТ. Во всех трех случаях отмечена хорошая переносимость, побочные реакции были легкой и умеренной степени тяжести. Наблюдалась тенденция к более высокой частоте локальных и системных реакций при введении вакцин внутрикожно. Уровень серопротекции на 28-й день после вакцинации при всех способах введения вакцины был сопоставим: к штамму A/H1N1 при внутримышечном введении – 89%, при интрадермальном – 80% и при внутрикожном – 82%; к штамму A/H3N2 – 75, 70 и 82% соответственно; к штамму гриппа В – 67,9, 36,7 и 53,6%.
A. Weinberg и соавт. [62] и D. Curtis и соавт. [63] изучили эффективность четырехвалентных вакцин у ВИЧ-инфицированных детей и молодых людей в возрасте до 25 лет и доказали сопоставимость развивающегося в ответ на вакцинацию Т-клеточного и В-клеточного иммунного ответа с результатами у пациентов без ВИЧ-инфекции того же возраста, а также безопасность применения этой вакцины при ВИЧ-инфекции.
Эффективность и безопасность вакцинации также изучали у ВИЧ-инфицированных детей и беременных. После применения трехвалентной вакцины от гриппа у ВИЧ-инфицированных детей (средний возраст – 7,2 года), получающих АРТ, были отмечены сероконверсия к штаммам A/H3N2, A/H1N1 и B в 73,9, 56,5 и 52,2% случаев соответственно, а также отсутствие прогрессирования ВИЧ-инфекции и развития гриппа в течение 3 мес. [35]. Безопасность и достаточную иммуногенность вакцины против гриппа у ВИЧ-инфицированных детей, а также сохранение защитного титра антител в течение 3 мес. также отмечали E. Tanzi и соавт. [64]. Снижение эффективности трехвалентной вакцины у ВИЧ-инфицированных детей в зависимости от количества CD4+-лимфоцитов продемонстрировали P. Kosalaraksa и соавт. [65].
По мнению D.J. Curtis и соавт. [66], на эффективность вакцинации существенно влияет возраст. Так, после вакцинации титр 1:40 и выше был отмечен у 23% детей в возрасте 4–9 лет, у 50% детей 9–18 лет и у 70% молодых людей в возрасте от 18 до 25 лет (p = 0,001). Madhi S.A. и соавт. [67] продемонстрировали низкую эффективность двукратной вакцинации трехвалентной неоадъювантной вакциной от гриппа ВИЧ-инфицированных детей (средний возраст – 23,8 мес.); уровень серопротекции к штаммам гриппа А/H1N1, А/H3N2 и B составил в этой группе всего 47,5, 50,0 и 40,0% соответственно; число заболевших гриппом в этой группе и в группе детей, получившими плацебо, было сопоставимо – 13 из 205 (6,3%) и 17 из 200 (8,5%) соответственно.
I.O. Okike и соавт. [68] отметили высокую (87%) иммуногенность и безопасность двукратной вакцинации адъювантной вакциной против гриппа A (H1N1) у ВИЧ-инфицированных детей в возрасте от 3 до 18 лет (средний возраст – 11,2 года). P. Palma и соавт. [69] продемонстрировали, что наилучшего эффекта можно достигнуть при двукратной вакцинации ВИЧ-инфицированных детей, применяя моновалентную вакцину A/H1N1 с адъювантом MF59. После 1-й дозы вакцины нарастание тира антител до 1:40 и выше отмечено у 60% детей, после 2-й – у 82%. Рядом зарубежных авторов изучен вопрос вакцинации от гриппа ВИЧ-инфицированных беременных. В России 1,5% беременных ВИЧ-инфецированы. У детей, рожденных ВИЧ-инфицированными матерями, повышен риск заболеваемости и смертности от инфекционных заболеваний, особенно в первые 6 мес. жизни, что частично может быть связано с более низким уровнем специфических защитных антител, приобретенных от матерей трансплацентарно [70]. Безопасность вакцинации от гриппа беременных доказана S.A. Madhi и соавт. [71] Известно, что концентрация защитных антител у младенцев по отношению к материнским антителам варьирует для разных антигенов в зависимости от срока беременности: как правило, от 75 до 135% при сроке 37 нед. и более, 50–95% в 33–36 нед. и 30–55% в 28–32 нед. беременности [72–74]. На протяжении последних лет изучается иммуногенность различных вакцин от гриппа у ВИЧ-инфицированных беременных во II–III триместре беременности. Установлено, что при однократной вакцинации трехвалентной вакциной против гриппа защитные титры антител формировались в 41% случаев против 92% у беременных без ВИЧ-инфекции и были тем выше, чем выше было количество CD4+-лимфоцитов (более 350 клеток/мкл) [75]. Двукратная вакцинация с введением увеличенной дозы антигена в моновалентной вакцине от гриппа H1N1 2009 увеличила число эффективно вакцинированных (превышение серопротекторного порога 1:40) до 80% после 2-й вакцинации [76]. Применение для вакцинации ВИЧ-инфицированных ASO3-адьювантных вакцин привело к увеличению доли эффективно вакцинированных до 70–93% после 1-й дозы и до 94–99% – после 2-й, что соответствует показателям у беременных без ВИЧ-инфекции [77–78].
Выводы
- У ВИЧ-инфицированных с тяжелой иммуносупрессией грипп протекает тяжелее, чаще развиваются осложнения и наблюдаются неблагоприятные исходы. С широким внедрением ВААРТ частота тяжелых форм гриппа у больных, получающих ее, стала сопоставима с таковой у пациентов без ВИЧ того же возраста. ВААРТ у ВИЧ-инфицированных не только замедляет течение основного заболевания, но и снижает риск развития тяжелой формы гриппа, в том числе пандемического.
- Для эффективного лечения гриппа у ВИЧ-инфицированных в течение первых 48 ч от начала заболевания необходимо применять осельтамивир.
- Предпочтительно вакцинировать ВИЧ-инфицированных двукратно адъювантной гриппозной вакциной после снижения ВН на фоне ВААРТ до неопределяемой и когда количество CD4+-лимфоцитов более 200 клеток/мкл (если это возможно).
- У ВИЧ-инфицированных пациентов иммуногенность вакцины снижается, когда репликация ВИЧ не контролируется ВААРТ и/или когда количество CD4+-лимфоцитов составляет менее 200 клеток/мкл. Продолжительность серопротекции у них короче, чем у неинфицированных лиц, что требует увеличения числа вакцинаций (бустерных доз).
- Живая аттенуированная противогриппозная вакцина противопоказана пациентам с тяжелым иммунодефицитом (количество CD4+-лимфоцитов менее 200 клеток/мкл).


