Заболевания, этиологически обусловленные вирусом гепатита В (HBV), широко распространены во всем мире. Более чем у 2 млрд человек выявляются серологические маркеры инфицирования, примерно у 360 млн отмечается хроническое течение HBV-инфекции, следствием чего может быть формирование хронического гепатита, цирроза и рака печени [1]. По мнению специалистов [2], можно выделить 2 разных сценария распространения HBV-инфекции в мире. Для высокоэндемичных стран Восточного региона характерно преобладание хронических форм инфекционного процесса, приводящих к высоким показателям смертности и инвалидизации; заражение HBV происходит преимущественно перинатальным путем или в раннем детском возрасте. На Западе чаще встречаются острые формы гепатита В (ГВ), завершающиеся выздоровлением (self-limiting HBV-infection). Инфицирование, как правило, происходит во взрослом возрасте, а длительное вирусоносительство HBV и формирование осложнений в общей популяции наблюдается относительно редко. Внедрение массовой иммунизации новорожденных позволило значительно снизить распространенность ГВ и его хронических форм в странах, где HBV-инфекция обусловлена инфицированием в раннем детстве. В то же время в индустриально развитых странах Запада основные усилия здравоохранения по профилактике ГВ были направлены преимущественно на предупреждение и регистрацию случаев и вспышек этой инфекции. Увеличение миграции из стран с высокой распространенностью HBV-инфекции привело к возрастанию заболеваемости острыми и хроническими формами ГВ в странах Западного региона [3, 4]. В этой связи значительные успехи как на Востоке, так и на Западе связывают с развитием программ иммунизации. Именно благодаря широкому внедрению программ вакцинопрофилактики в борьбе с ГВ были достигнуты существенные успехи, которые позволили рассматривать возможность элиминации и эрадикации HBV-инфекции [5].
В то же время, несмотря на 25-летний опыт проведения иммунизации против ГВ, многие вопросы, связанные с длительностью протективного иммунитета и защищенностью вакцинированных лиц, все еще остаются нерешенными. Так, на последнем заседании Совета по профилактике вирусных гепатитов (Viral Hepatitis Prevention Board), прошедшем в Милане в ноябре 2011 г. [6], была подтверждена возможность возникновения различных вариантов HBV-инфекции у лиц, ранее привитых против ГВ (по результатам сероэпидемиологических исследований, проведенных в разных странах). В связи с этим было предложено стандартное определение термина «прорыв инфекции» (breakthrough infection) – наличие HBV-инфекции у привитых надлежащим образом лиц, подтвержденное серологическими тестами (в подавляющем большинстве случаев единственным маркером инфицирования были антитела к коровому антигену HBV – анти-НВс). Были определены и причины прорыва инфекции: неудачная (failure) вакцинация; инфекция у детей, рожденных HBsAg-позитивными матерями; появление вирусных мутаций и др. [6].

Как правило, у привитых против ГВ лиц случаи клинически выраженных форм гепатита не отмечаются. В то же время в ряде исследований, проведенных в регионах с различной распространенностью инфекции, было показано наличие серологических маркеров, свидетельствующих о заболевании у вакцинированных. Так, в Гамбии через 15 лет иммунизации против ГВ защитный уровень анти-HBs был определен только у 13,8% подростков, и в то же время у 10,1% обследованных были выявлены анти-НВс и у 0,7% – HBsAg [7]. В работе польских исследователей установлено, что у 4,5% подростков, вакцинированных при рождении, обнаруживали анти-НВс, а у 1,5% из них выявляли также HBsAg [8]. Через 24 года после вакцинации, начатой при рождении, частота обнаружения анти-HВs составила 30,2%, HBsAg – 1,0%, анти-НВс – 6,7%. У 19 из 24 лиц с анти-НВс была выявлена ДНК HBV, что послужило свидетельством наличия латентной (occult) HBV-инфекции. Также заслуживает внимания тот факт, что у одного из обследованных через 4 года после обнаружения анти-НВс был выявлен HBsAg [9]. В этой связи необходимо отметить, что латентная HBV-инфекция у вакцинированных детей не является редким случаем. По литературным данным, частота этой формы инфекционного процесса у привитых детей может быть в пределах от 0 до 10,9%, а среди детей, рожденных HBsAg-позитивными матерями, достигать 28% [10, 11].
Единого мнения о причинах возникновения HBV-инфекции у привитых до настоящего времени нет. В частности рассматривается роль вирусных мутаций, иммунокомпетентности макроорганизма, ослабления иммунной памяти и др. Нередко выявление маркеров HBV-инфекции связывают со снижением иммунного ответа на вакцинацию с течением времени. По данным A. Aghakhani и соавт. [12], у 24% детей, после завершения вакцинации которых прошло 15 лет, отсутствовали анти-HВs на защитном уровне. Это дало основание авторам рекомендовать введение бустер-дозы в регионах с низкой эндемичностью HBV-инфекции. В то же время научные доказательства, подтверждающие абсолютную необходимость проведения широкой бустер-иммунизации, до сих пор не получены. Полагают, что в регионах с высокой распространенностью HBV-инфекции бустер-иммунизация не является нецелесообразной, поскольку для этих территорий характерна естественная бустер-реакция (natural booster response) – нарастание уровня анти-HBs без ревакцинации, происходящее за счет «контактов» с вирусом на фоне достаточного уровня протективных антител. Это способствует усилению иммунного ответа при отсутствии определяемых маркеров инфекции, в частности анти-НВс [6].
 Таким образом, неоднозначность современных научных данных в отношении факторов, влияющих на эффективность вакцинации против ГВ, свидетельствует об актуальности дальнейшего изучения этого вопроса в регионах с разной распространенностью HBV-инфекции.
Таким образом, неоднозначность современных научных данных в отношении факторов, влияющих на эффективность вакцинации против ГВ, свидетельствует об актуальности дальнейшего изучения этого вопроса в регионах с разной распространенностью HBV-инфекции.
Цель работы – определение показателей иммунного ответа на вакцинацию против ГВ в разные сроки после иммунизации и оценка ее эпидемиологической эффективности на основании частоты обнаружения маркеров HBV-инфекции у привитых и непривитых детей из разных регионов Украины.
Материалы и методы
В работе использован комплексный подход, объединяющий методику кросс-секционного (одномоментного, поперечного) серологического и описательного исследования [13]. В группу обследованных вошли 1586 привитых и непривитых против ГВ детей из пяти областей Украины, относящихся к разным географическим территориям: Югу (428), Северу (605), Западу (194) и Центру (359). Серологические исследования проведены методом иммуноферментного анализа (ИФА) на сертифицированном оборудовании с использованием коммерческих тест-систем производства НПК АОЗТ «ДіаПрофМед» и ООО «МедБіоАльянс» (Украина). В образцах сывороток крови определяли поверхностный антиген HBV (HBsAg), антитела к HBsAg (анти-HBs) и суммарные (total) антитела к коровому антигену HBV (анти-НВс). Первично реактивные результаты выявления HBsAg подтверждали с использованием коммерческих тест-систем тех же производителей, предназначенных для верификации позитивных результатов тестирования. Для оценки количественных показателей иммунного ответа у привитых детей определяли концентрацию анти-HBs. В соответствии с инструкцией производителя тест-систем, негативные по результатам исследования на анти-HBs образцы считали такими, которые не содержат антител на минимальном защитном уровне (<10 МЕ/л); при концентрации анти-HBs ≥ 100 МЕ/л уровень иммунного ответа считали высоким.
Группы обследованных детей и объем проведенных исследований представлены в табл. 1.
О каждом обследованном ребенке была получена следующая информация: дата рождения, пол, прививочный анамнез. Для привитых детей – даты проведения вакцинаций против ГВ и использованная вакцина. Забор крови осуществлялся медицинским персоналом в региональных учреждениях здравоохранения с информированного согласия родителей. Все статистические данные об обследованных детях и результаты их тестирования на серологические маркеры были внесены в компьютерную базу данных.
Коэффициент эпидемиологической эффективности вакцинации определяли по формуле: КЭ = [(В – А)/В] × 100, где КЭ – коэффициент эффективности (%), А – частота выявления маркеров HBV-инфекции у привитых, В – частота выявления маркеров HBV-инфекции у непривитых [14].
При анализе результатов серологических исследований цифровые данные выражали в относительных величинах – (P ± mp), %; среднюю ошибку, 95% доверительный интервал (95% ДИ) и достоверность различий между показателями определяли стандартными статистическими методами с использованием программы Microsoft Office Excel [15, 16].
Результаты и обсуждение
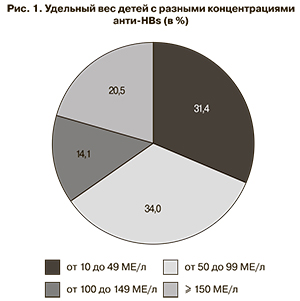
Результатами серологических исследований было установлено, что в 567 (53,3 ± 1,5%) из 1063 образцов сывороток крови детей, привитых против ГВ по полной схеме, регламентируемой календарем профилактических прививок Украины (0–1–6 мес), обнаружены специфические антитела к HBsAg в защитных концентрациях (≥ 10 МЕ/л). Соответственно у 496 (46,7 ± 1,5%) обследованных детей уровень анти-HBs был ниже минимального защитного. Анти-HBs в высокой (≥ 100 МЕ/л) концентрации были выявлены всего у 196 (18,4 ± 1,2%) вакцинированных детей. Наиболее часто у привитых детей с защитным уровнем антител концентрация анти-HBs находилась в пределах 10–49 и 50–99 МЕ/л (рис. 1).
Среди детей, обследованных через 10 лет после вакцинации, удельный вес серонегативных в отношении анти-HBs составлял 63,0 ± 9,3%, среди детей, после вакцинации которых прошло 5 лет – 46,4 ± 4,4%, среди детей, привитых за 1 год до обследования – 31,8 ± 4,5%. Удельный вес детей с отсутствием защитного уровня анти-HBs через 10 лет после вакцинации был достоверно выше, чем среди детей, иммунизированных за 1 год до проведенного обследования (р < 0,05).
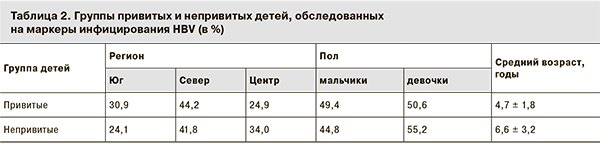
Связь между наличием анти-HBs в защитных концентрациях у привитых детей и защищенностью от заболевания окончательно не установлена. Однако если не проводится поствакцинальный иммунологический контроль, невозможно определить, является ли негативный результат тестирования на анти-HBs следствием снижения напряженности иммунитета или отсутствия иммунного ответа на вакцину. По этой причине установление удельного веса лиц с защитным уровнем антител в разных возрастных группах может быть единственной возможностью, позволяющей определить прослойку защищенных от инфекции в изучаемых группах. Нами было обследовано 326 детей в возрасте 1–4 года, 612 – в возрасте 5–9 лет и 121 – возрасте 10–14 лет. Распределение детей с разными концентрациями анти-HBs в этих возрастных группах представлено на рис. 2. Наибольший удельный вес детей с уровнем анти-HBs ниже 10 МЕ/л был зарегистрирован в возрастной группе 10–14 лет – 57,9 ± 4,5%, среди детей 1–4 лет он составил 39,3 ± 2,7%, 5–9 лет – 48,7 ± 2,0%.
С учетом полученных данных о значительной доле детей с низкими титрами анти-HBs среди 10–14-летних, мы сравнили показатели выявления маркеров инфицирования HBV у привитых (n = 107) и непривитых (n = 98) детей в этой возрастной группе. Среди вакцинированных детей маркеры HBV-инфекции были обнаружены у 4 обследованных (3,7 ± 1,8%), при этом у 3 из них отсутствовали анти-HBs, а у 1 уровень этих антител составил всего 35 МЕ/л. Среди непривитых детей двое (2,0 ± 1,4%) имели серологический профиль «анти-НВс+/анти-HBs+», при этом у 1 из них в анамнезе был перенесенный ГВ. Таким образом, серологические находки маркеров инфицирования HBV у 10–14-летних привитых детей сопровождались отсутствием (или утратой) анти-HBs на протективном уровне, а показатели распространенности HBV-инфекции были сопоставимы с таковыми у непривитых детей того же возраста.
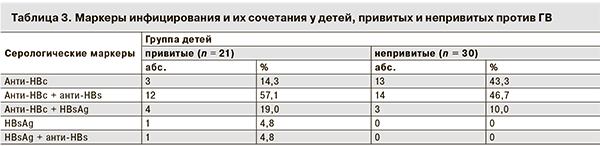
Дискуссионным остается вопрос, зависит ли напряженность иммунного ответа на вакцинацию против ГВ от начала ее проведения. По данным некоторых исследователей [6, 17, 18], протективные антитела быстрее исчезают у тех, кто был вакцинирован при рождении, по сравнению с детьми, иммунизированными в более старшем возрасте. Подавляющее большинство детей в Украине прививают в течение первых суток после рождения. Среди обследованных нами доля таких детей составила 67,4 ± 1,4%, доля привитых на протяжении 1-го года жизни – 23,0 ± 2,7%, привитых в возрасте старше 1 года – 9,6 ± 0,9%. Через 5 лет после окончания полного курса вакцинации защитный уровень анти-HBs был определен у 42 (46,7 ± 5,3%) из 90 обследованных детей, привитых при рождении, у 12 (66,7 ± 11,1%) из 18 детей, привитых в 1-й год жизни, и у 14 (77,8 ± 9,8%) из 18 детей, привитых в возрасте старше 1 года (рис. 3). Разница между удельным весом детей с протективным уровнем антител среди привитых в возрасте старше 1 года и привитых при рождении статистически достоверна (р < 0,01). Обращает на себя внимание тот факт, что в группе детей, привитых в возрасте старше 1 года, был наименьший удельный вес серонегативных в отношении анти-HBs – 22,2 ± 9,8% и наибольший удельный вес лиц с высоким уровнем анти-HBs – 33,3 ± 11,1%. Среди детей, привитых при рождении, высокие концентрации анти-HBs отмечены лишь у 6,7 ± 2,6% (р < 0,05).
На следующем этапе сравнивали уровень иммунного ответа на вакцинацию против ГВ в зависимости от кратности ее проведения. Как и следовало ожидать, наибольший удельный вес серонегативных (анти-HBs < 10 МЕ/л) был среди детей, получивших только 1 дозу вакцины – 74,2 ± 5,6%, в то время как среди детей, получивших 2 и 3 дозы вакцины, доля таких лиц статистически не отличалась – 50,0 ± 5,3% и 46,7 ± 1,5% соответственно. При сравнении концентраций анти-HBs у детей, привитых по полной схеме и получивших только 2 дозы вакцины, не было установлено различий: высокий уровень иммунного ответа (≥ 100 МЕ/л) отмечен у 18,4 ± 1,2% детей, привитых трижды, и у 17,0 ± 4,0% детей, получивших 2 дозы вакцины; анти-HBs в концентрации 10–99 МЕ/л – у 34,9 ± 1,5% и у 33,0 ± 4,0% соответственно. Полученные данные согласуются и подтверждают результаты исследований, свидетельствующих о сопоставимой иммунологической эффективности вакцинации против ГВ тремя и двумя дозами вакцины [6, 19, 20].
С нашей точки зрения, эпидемиологическую эффективность вакцинации против ГВ детей наиболее целесообразно рассчитывать на основании показателей не заболеваемости, а инфицированности привитых и непривитых лиц. Это связано с тем, что в настоящее время в Украине показатели регистрируемой заболеваемости острыми формами ГВ у детей до 14 лет не превышают 2 случаев на 100 тыс. детей соответствующей возрастной группы. Исходя из этого, мы провели обследование на наличие серологических маркеров HBV-инфекции 914 детей, получивших полный курс вакцинации, и 373 непривитых детей. Уровни инфицированости сравнивали у привитых и непривитых детей из разных регионов Украины. Распределение сравниваемых групп детей по территориям, полу и возрасту представлено в табл. 2.
В целом у 21 (2,3 ± 0,5%) ребенка из группы привитых были обнаружены серологические маркеры инфицирования HBV, в то время как среди непривитых – у 30 (8,0±1,4%), что было достоверно выше (р < 0,005). Возрастных различий между привитыми и непривитыми детьми с наличием маркеров HBV-инфекции не установлено (средний возраст 6,8 и 6,4 года соответственно). Таким образом, с учетом распространенности маркеров инфицирования HBV среди привитых и непривитых против ГВ детей, по данным многоцентрового исследования, коэффициент эпидемиологической эффективности вакцинации против ГВ в Украине составил 71,2%.
Анализируя результаты обследования привитых и непривитых детей, можно констатировать, что преимущественным маркером инфицирования HBV в обеих группах детей были антитела к коровому антигену – анти-НВс. Самостоятельно и в сочетании с анти-HBs эти антитела определялись соответственно у 71,4 ± 9,9% и 90,0 ± 1,5% инфицированных детей. Серологические профили, которые были выявлены у привитых и непривитых детей, инфицированных HBV, представлены в табл. 3.
 Наиболее часто у детей с маркерами инфицирования встречался серологический профиль анти-НВс+/анти-HBs+ – у 57,1 ± 10,8% привитых и у 46,7 ± 9,1% непривитых против ГВ детей. В группе непривитых детей такой серологический профиль может быть свидетельством перенесенной инфекции, а у привитых – указывать на так называемый контакт с вирусом. У 8 (66,7%) из 12 привитых детей с наличием анти-НВс и анти-HBs одновременно были высокие (≥150 МЕ/л) титры анти-HBs, что можно расценивать как активацию иммунной памяти в ответ на инфицирование HBV. Среди обследованных детей лишь у 1 непривитого ребенка в анамнезе был перенесенный за год до обследования острый ГВ, и серологический профиль анти-НВс+/анти-HBs+ вероятнее всего, соответствовал стадии поздней реконвалесценции.
Наиболее часто у детей с маркерами инфицирования встречался серологический профиль анти-НВс+/анти-HBs+ – у 57,1 ± 10,8% привитых и у 46,7 ± 9,1% непривитых против ГВ детей. В группе непривитых детей такой серологический профиль может быть свидетельством перенесенной инфекции, а у привитых – указывать на так называемый контакт с вирусом. У 8 (66,7%) из 12 привитых детей с наличием анти-НВс и анти-HBs одновременно были высокие (≥150 МЕ/л) титры анти-HBs, что можно расценивать как активацию иммунной памяти в ответ на инфицирование HBV. Среди обследованных детей лишь у 1 непривитого ребенка в анамнезе был перенесенный за год до обследования острый ГВ, и серологический профиль анти-НВс+/анти-HBs+ вероятнее всего, соответствовал стадии поздней реконвалесценции.
Серологические признаки текущей активной HBV-инфекции (HBsAg+/анти-НВс+) были обнаружены у 3 (10,0%) непривитых и 4 (19,0%) привитых против ГВ детей, при этом в сыворотках крови привитых детей отсутствовали анти-HBs, что может быть следствием либо утраты иммунитета, либо неудачи вакцинации.
Текущая латентная инфекция (по наличию «изолированных анти-НВс») была обнаружена у 14,3% инфицированных привитых детей. У 2 привитых детей был выявлен HBsAg при отсутствии суммарных анти-НВс, что по серологическим признакам может свидетельствовать о недавнем инфицировании. У 1 ребенка присутствие HBsAg сопровождалось наличием анти-HBs в низкой (23 МЕ/л) концентрации, что, предположительно, не исключает инфицирования мутантным штаммом HBV.
В целом у 8 (0,9%) из всей группы обследованных привитых детей обнаружены маркеры HBV-инфекции при отсутствии анти-HBs. Эти случаи следует рассматривать как прорыв инфекции, причиной которого могла быть неудачная вакцинация или утрата иммунитета на момент обследования.
В соответствии с критериями ВОЗ [21], Украина может быть отнесена к регионам с невысокой распространенностью HBV-инфекции, в которых, как установлено, вероятность естественной бустер-иммунизации невелика, при этом наибольшему риску инфицирования подвергаются лица в возрасте 15–30 лет. В этой связи и с учетом полученных в нашей стране данных, вопрос о необходимости и целесообразности проведения поствакцинальной оценки иммунологической эффективности вакцинации против ГВ и(или) проведении серологического мониторинга за напряженностью иммунитета в целевых группах населения актуален для регионов с невысокой распространенностью НВV-инфекции.
Выводы
- Установлено, что у 46,7 ± 1,5% детей, привитих против ГВ, отсутствовали протективные антитела. Анти-HBs в высокой (> 100 МЕ/л) концентрации обнаружены всего у 18,4 ± 1,2% вакцинированных детей.
- Удельный вес серонегативных в отношении анти-HBs детей среди привитых зависел от срока, прошедшего после вакцинации, и составлял 63,0% среди детей, вакцинированных за 10 лет до обследования, и 31,8% – среди привитых за 1 год до обследования.
- Установлено, что доля серонегативных и детей с низкими и высокими концентрациями анти-HBs у привитых против ГВ трехкратно и двукратно статистически не различалась. В то же время удельный вес серонегативных среди детей, получивших только 1 дозу вакцины, был достоверно выше, чем среди вакцинированных по полной схеме.
- Показана более высокая иммунологическая эффективность вакцинации против ГВ, проведенной в возрасте старше 1 года, по сравнению с вакцинацией новорожденных. Защитный уровень анти-HBs был определен у 46,7 ± 5,3% детей, привитых при рождении, и у 77,8 ± 9,8% иммунизированных в возрасте старше 1 года. Среди вакцинированных в возрасте старше 1 года также был больше удельный вес детей с высокими концентрациями анти-HBs.
- Частота выявления серологических маркеров HBV-инфекции составила 2,3 ± 0,5% у детей, полностью привитых против ГВ, и 8,0 ± 1,4% – у непривитых детей. У 8 (0,9%) из 914 обследованных привитых детей были выявлены маркеры инфицирования HBV на фоне негативного результата тестирования на анти-HBs, что может быть расценено как «прорыв инфекции». На момент обследования клинические признаки гепатита у инфицированных детей отсутствовали.
- Коэффициент эпидемиологической эффективности вакцинации против ГВ в Украине, рассчитанный на основании показателей инфицированности привитых и непривитых детей, составил 71,2%.
- Вопрос о необходимости бустер-вакцинации против ГВ требует дальнейшего изучения. На небольшом количестве исследований нами были получены данные о сопоставимом уровне инфицированности как привитых, так и непривитых детей в возрасте 10–14 лет – 3,7 ± 1,8% и 2,0 ± 1,4% соответственно. Практически у всех привитых детей наличие маркеров HBV-инфекции сопровождалось отсутствием защитного уровня анти-HBs.


