Крымская геморрагическая лихорадка (КГЛ) – зоонозная природно-очаговая инфекционная болезнь (код по МКБ10 А98.0), вызываемая вирусом Крымской-Конго геморрагической лихорадки (ККГЛ).
Вопросы вирусологии, эпидемиологии и профилактики, клиники и диагностики КГЛ были рассмотрены в наших предыдущих статьях [1, 2]. Показано, что в XXI веке активизировались старые и появились новые природные очаги КГЛ в Евразии, существенно возросла заболеваемость. В этой ситуации особое значение приобретает оптимизация лечения КГЛ, разработка и внедрение новых терапевтических средств, основанных на фундаментальных исследованиях и глубоком понимании патогенеза этой тяжелой инфекции. В этом сообщении обсуждаются молекулярные и клеточные механизмы, лежащие в основе клинических проявлений КГЛ, и перспективы патогенетической и этиотропной терапии геморрагических лихорадок. Поскольку результаты исследований, проведенных в России, хорошо известны читателю и доступны из других источников, в обзоре особое внимание уделено анализу англоязычной литературы.
Патогенез КГЛ
Первой фазой патогенетического процесса является проникновение вируса в кровь. Рецепторы, через которые вирус ККГЛ связывается с клетками, и механизмы его интернализации неясны. Возможно, клеточным рецептором является нуклеолин, экспрессирующийся на поверхности макрофагов, эндотелиальных клеток, гепатоцитов и взаимодействующий с Gc, гликопротеином вируса ККГЛ [3, 4]. Показано, что повреждение цитоскелета и/или ингибирование клатрин-зависимого эндоцитоза значительно снижает эффективность инфицирования тканевых клеток вирусом ККГЛ in vitro [5, 6]. Последующее размножение вируса ККГЛ происходит в цитоплазме гепатоцитов, эндотелиальных и эпителиальных клеток, а также в дендритных клетках моноцитарного происхождения, но не в самих клетках крови [7–9].
Поскольку характерными клиническими проявлениями КГЛ являются геморрагии и повышение проницаемости сосудов, логично, отчасти по аналогии с другими геморрагическими лихорадками, предположить, что при КГЛ имеет место гиперактивация и повреждение эндотелиальных клеток [10]. В пользу данной гипотезы свидетельствует то, что при КГЛ сывороточные концентрации таких макромолекул, как фактор роста васкулярного эндотелия (VEGF), растворимые молекулы клеточной адгезии ICAM-1 и VCAM-1, растворимые селектины, фактор ингибирования миграции макрофагов (MIF), растворимый рецептор урокиназоподобного активатора плазминогена suPAR и т. п. значимо отличны от нормальных [11–13]. Теоретически возможны два механизма действия вируса: 1) непосредственно на клетки эндотелия; 2) опосредованно путем активации лейкоцитов, секретирующих провоспалительные цитокины (ФНО, IL-6, IL-10) и цитотоксические молекулы. В опытах in vitro реализуются оба способа, но их относительная значимость in vivo неясна [8, 9]. Вирус ККГЛ способен также, размножаясь в культуре клеток гепатоцитов, индуцировать явление «стресса эндоплазматического ретикулума» и тем самым активировать «внутренний», митохондриально-зависимый путь апоптоза, чему в клинических условиях соответствовало бы поражение печени [7]. Апоптоз играет двойную и противоречивую роль в патогенезе вирусных инфекций. В культуре клеток альвеолярного эпителия человека А549 заражение вирусом ККГЛ через 48–72 ч приводит к апоптозу, опосредованному активацией каспазы-3 (цистеин-аспартат-специфической протеазы). При этом размножение вируса к 48-му часу почти на порядок слабее, чем в клетках, лишенных каспазы-3, или в клетках, в которых апоптоз, зависимый от каспазы-3, ингибирован. Характерно, что при этом активация каспазы-3 приводит к расщеплению нуклеопротеина по сайту DEVD, присутствующему во всех известных штаммах вируса ККГЛ [14]. В этом смысле апоптоз играет роль защитного механизма, ограничивающего распространение инфекции. С другой стороны, массовая гибель клеток и деструкция тканей являются элементом патологического процесса и наблюдаются, например, при летальных заболеваниях геморрагической лихорадкой Эбола [10]. Не исключено, что зараженные вирусом ККГЛ клетки уничтожаются также цитотоксическими лимфоцитами или натуральными киллерами по механизму антителозависимой клеточной цитотоксичности, поскольку моноклональные антитела к Gn, гликопротеину оболочки вируса ККГЛ, не нейтрализуют вирус в культуре, но защищают мышей от летальной дозы вируса ККГЛ [15].
Размножение вируса в клетке ограничивается комплексом интерферон-зависимых механизмов. С другой стороны, многие высоковирулентные вирусы приобрели способность частично или полностью препятствовать действию этих механизмов [16, 17]. Вирусные двухцепочечные РНК и трифосфорилированная одноцепочечная РНК распознаются в инфицированной клетке цитоплазматическими сенсорами – RIG-I-подобными геликазами (собственно RIG-I и MDA-5), что приводит к фосфорилированию интерферон-регулирующего фактора 3 (IRF-3). IRF-3 транспортируется в ядро клетки и во взаимодействии с другими факторами (киназой PKR, зависимой от двухцепочечной РНК, и транскрипционным фактором NF-κB) включает синтез интерферонов. Эта «первая волна» интерферонов активирует интерферон-регулирующий фактор 7 (IRF-7), стимулирующий выброс более мощной и разнообразной «второй волны» интерферонов [18].
Показано, что интерфероны типа I (ИФН-α, более 13 субтипов, и ИФН-β, единственный субтип) ингибируют репликацию вируса ККГЛ in vitro, если добавляются к культуре клеток до или одновременно с заражением вирусом. В исследовании [19] было сопоставлено действие Роферона А (рекомбинантного интерферона субтипа α-2a), Интрона А (рекомбинантного интерферона субтипа α-2b) и Мультиферона (смеси естественных ИФН-α различных субтипов). Все эти агенты, добавленные в концентрации от 10 до 1000 Ед/мл к культуре клеток человеческого альвеолярного эпителия А549 за 24 ч до заражения вирусом ККГЛ, дозозависимо угнетают его репликацию, причем эффект Мультиферона на порядок выше, чем эффект Роферона А или Интрона А. Максимальный эффект достигался при концентрации Мультиферона 1000 Ед/мл: число копий вируса ККГЛ в супернатанте после 48 ч культивирования было приблизительно в 1000 раз ниже, чем без добавления интерферона.
Напротив, репликация вируса ККГЛ в клетках существенно замедляет выработку ими собственных интерферонов и делает клетки менее чувствительными к ИФН-α, добавляемому извне [20].
Интерфероны типа I и II (ИФН-γ), находящиеся внеклеточно, связываются с клетками млекопитающих через специфические рецепторы, которые соответственно называются интерферон-рецепторы типа I и II [21]. Показано, что у взрослых мышей, генетически лишенных интерферон-рецепторов типа I (IFNAR-/-линия мышей), через 42–70 ч после интраперитонеального введения вируса ККГЛ (от 10 до 106 вирусных единиц) развиваются выраженные патологические процессы, приводящие к гибели на 2–4-е сутки [22]. При этом вирусная нагрузка достигает 108 копий генома вируса ККГЛ на мл крови и 1011 копий на г тканей (печени, селезенки, почек); наблюдаются геморрагии в печени. У контрольных мышей вирусная нагрузка через 2 сут после заражения на 3–4 порядка ниже, на 3-и сутки и позже вирус не выявляется. Летальность, выраженные проявления инфекции и патоморфологические изменения органов отсутствуют [22].
Вслед за связыванием интерферонов с рецепторами активируются «янус-киназы» JAK-1 и TYK-2, фосфорилирующие «белки, преобразующие сигнал и активирующие транскрипцию» – STAT1 и STAT2, которые вместе с IRF-9 образуют комплексы ISGF-3. Комплексы перемещаются в ядро и связываются с промоторными участками генов, стимулируемых интерферонами (IFN-stimulated genes или ISG) [21]. Среди продуктов генов ISG известны протеинкиназа PKR, ГТФ-азы семейства Mx, 2’,5’-олигоаденилат синтетаза (2’,5’-OAS), виперин, РНК-специфическая аденозиндезаминаза (ADAR1), продукты генов ISG20 и ISG56. Предполагается, что в защите от вируса ККГЛ задействованы как минимум ГТФ-аза MxA, связывающаяся с нуклеопротеином, эндонуклеаза ISG20, расщепляющая одноцепочечную РНК, и PKR, включающая блокаду трансляции вирусной и клеточной мРНК. В свою очередь вирус ККГЛ уклоняется от атаки, минимизируя взаимодействие с MDA-5 и особенно с RIG-I, а также частично ингибируя активацию IRF-3, NF-κB и, возможно, механизм JAK-STAT [4, 18].
У мышей линии STAT129 белок STAT1 отсутствует. После интраперитонеального заражения взрослых мышей этой линии вирусом ККГЛ (от 10 до 1000 КОЕ) 100% животных погибали на 5-й день [23]. При этом симптомы и лабораторные показатели на 2–4-й день инфекции были сходны с таковыми у людей: лихорадка, видимая слабость, лейкопения, тромбоцитопения, повышение уровня АЛТ (в 10 раз выше нормы), резко повышенный уровень ИФН-α, ИФН-β и ИФН-γ и провоспалительных цитокинов TNF, IL-6, IL-10, IL-1β, CCL2. Примечательно, что неврологические нарушения – атаксия, парезы, параличи – отсутствовали. Вирусная нагрузка в крови мышей STAT129 повышалась в среднем от 6х106 копий/мл в 1-й день до 7х109 копий/мл на 3-й день. Начиная со 2-го дня вирус в высоких концентрациях обнаруживался в печени, селезенке и легких, с 3-го дня – в меньших концентрациях также в тканях почек и мозга. В печени наблюдались множественные очаги гепатоцеллюлярного некроза, в селезенке – признаки гибели фолликулярных лимфоцитов; ткани легких, почек и мозга выглядели гистологически не измененными. У контрольных мышей вирусная нагрузка падала от 103 копий/мл в 1-й день до нуля на 2–3-й день; органы были не повреждены и вирус в них практически не выявлялся.
Терапия рибавирином (100 мг/кг/день), начатая через 1 или даже через 24 ч после заражения, предотвращала гибель всех мышей STAT129, которым было введено 10 КОЕ вируса ККГЛ. При введении 1000 КОЕ вируса ККГЛ терапия, начатая через 1 ч, приводила к излечению 60% мышей и существенно замедляла гибель оставшихся 40% [23]. Авторы полагают, что данная лабораторная модель соответствует ранней (до появления специфических IgM-антител) стадии КГЛ у человека и имеет явные преимущества перед моделью новорожденных мышей, у которых вирус накапливается в мозгу, вызывая гибель от неврологических нарушений.
Подобные исследования, равно как и теоретические соображения, указывают, что концентрация вируса в крови может отражать и обусловливать тяжесть заболевания и прогноз развития КГЛ. В классических исследованиях А.М. Бутенко и М.П. Чумакова [24] вирус ККГЛ выделялся путем заражения новорожденных мышей из образцов крови 7 больных КГЛ в титре от 104 до 106 ЛД50/ мл в 1–4-й день заболевания и из образцов крови 7 больных в титре от 10 до 105 (в среднем 500 ЛД50/ мл) на 4–9-й день заболевания. После 12-го дня болезни вирус ККГЛ в крови не обнаруживался. По данным изучения 19 ростовских и болгарских больных в 1968 г., титр вируса ККГЛ в крови варьировал от 3х103 до 3х106 ЛД50/мл (в среднем 105 ЛД50/мл) на первой неделе заболевания. У 12 больных из Средней Азии вирусная нагрузка была чуть выше: в среднем 6х105 ЛД50/мл, диапазон – от 104 до 6х107 [25].
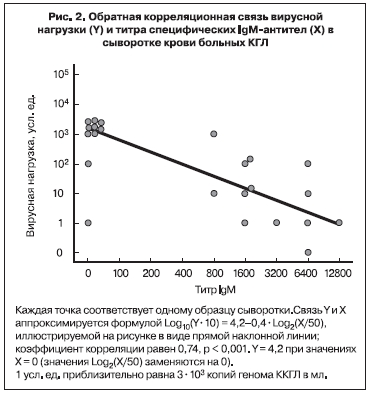
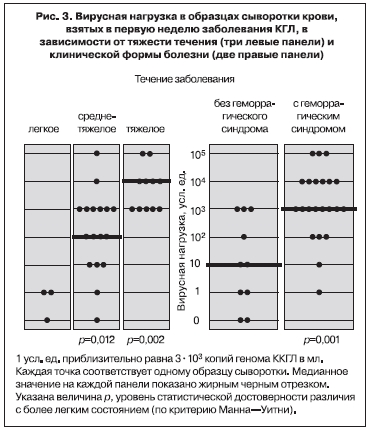
Нами в 2002 г. впервые была предпринята попытка оценить вирусную нагрузку в крови больных КГЛ методом ПЦР [26]. 51 образец сыворотки крови от 34 больных с верифицированным диагнозом КГЛ в Ставропольском крае был изучен в десятикратных серийных разведениях (от неразведенной до разведенной в 1 000 000 раз). Величина вирусной нагрузки в усл. ед. принималась равной величине последнего ПЦР-положительного разведения. С учетом чувствительности лабораторной методики 1 усл. ед. соответствовала приблизительно 3х103 копий генома вируса ККГЛ на мл. Вирусная нагрузка была максимальной (в среднем 3х107 копий/мл) в первые 4 дня болезни, уменьшалась по мере появления специфических IgM антител к концу 1-й недели, на 3-й неделе заболевания титр IgM достигал максимума, а РНК вируса ККГЛ более не выявлялась (рис. 1). Анализ показал сильную обратную корреляционную связь между вирусной нагрузкой и титром специфических IgM (рис. 2). В 1-ю неделю заболевания вирусная нагрузка была на 2 порядка выше у больных со среднетяжелым течением болезни, чем у больных с легким течением. У больных с тяжелым течением вирусная нагрузка была максимальной – от 3х106 до 3х108 копий/мл, то есть в среднем в 100 раз больше, чем у больных со среднетяжелым течением КГЛ (рис. 3). Вирусная нагрузка также была существенно выше у больных с геморрагическим синдромом по сравнению с больными, у которых он не развивался (см. рис. 3).
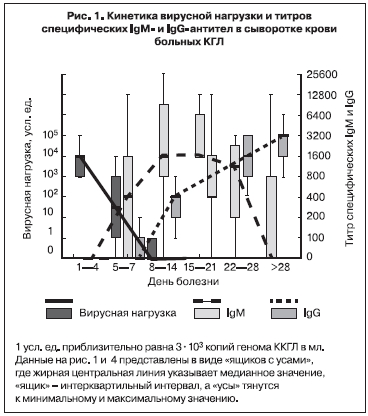
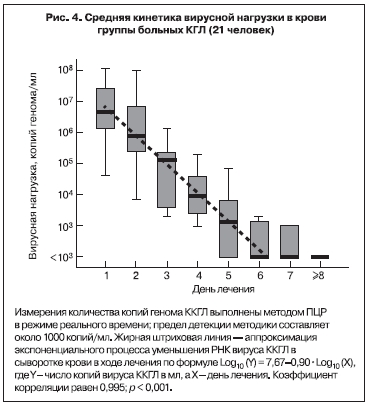
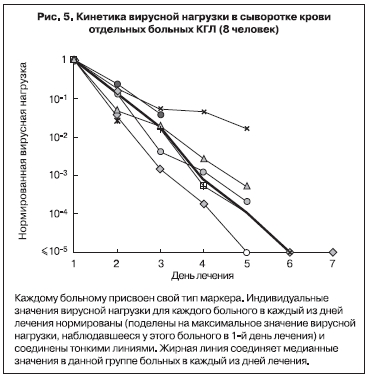
В 2003 г. кинетика вирусной нагрузки в крови больных КГЛ была изучена более детально с помощью количественной ПЦР в режиме реального времени [2]. В исследование было включено 80 проб сыворотки от 21 больного КГЛ из Ставропольского края. Больные были госпитализированы на 1–6-й день заболевания (в среднем на 3-й день). Вирусную нагрузку определяли у каждого больного ежедневно. Она была максимальной в 1-й день госпитализации, составляя в среднем (медианное значение) 4,8х106 копий/мл сыворотки, при этом минимальное значение равнялось 4000 копий/мл, а максимальное достигало 1,1х108 копий/мл. В процессе лечения последовательно снижались как величина средней вирусной нагрузки (рис. 4), так и значения вирусной нагрузки у отдельных больных (представительные кривые приведены на рис. 5). Данный факт показал, что при благоприятном исходе заболевания размножение вируса ККГЛ в крови происходит в основном в инкубационном периоде и на ранней стадии заболевания. Кинетика снижения в среднем была экспоненциальной, время полувыведения равнялось 8,0 ч, что соответствовало снижению в 8,0 раз за сутки. Вследствие этого через неделю лечения у большинства больных РНК вирус ККГЛ в крови более не выявлялся, заболевание закончилось выздоровлением [26, 27].
Документированная в наших исследованиях генетическими методами высочайшая вирусная нагрузка в крови ряда больных КГЛ, не наблюдающаяся при большинстве вирусных инфекций, в частности других арбовирусных лихорадках и геморрагической лихорадке с почечным синдромом [28], исчерпывающе объясняет высокую контагиозность КГЛ при прямом контакте с инфицированным материалом и опасность внутрибольничного инфицирования медицинских работников вирусом ККГЛ.
Зарубежные клинические исследования также свидетельствуют о высокой вирусной нагрузке в крови больных КГЛ и ее патогенетическом значении. По данным турецких исследователей, вирусная нагрузка при поступлении [на 2–10-й день болезни (в среднем на 5–6-й)] составляла от 103 до 109 копий/мл плазмы (в среднем 4х106 копий/мл) в группе из 27 выздоровевших больных, но среди 9 умерших пациентов была в 90% случаев от 109 до 1010 копий/мл. После госпитализации вирусная нагрузка снижалась приблизительно экспоненциально, к 5-му дню лечения в среднем до 10 копий/мл у выздоровевших больных и до 104 копий/мл при летальных исходах [29].
Вирусная нагрузка выше 109 копий/мл была статистически значимо связана с такими клиническими и лабораторными проявлениями крайне тяжелого течения болезни, как сопорозное состояние, кровотечения и кровоизлияния (особенно экхимозы), тромбоцитопения (менее 20х109 тромбоцитов/л) и другие признаки ДВС-синдрома на стадии гипокоагуляции, повышенный уровень АЛТ (> 700 Ед/л) и АСТ (> 900 Ед/л) [29].
В работе [30] также было показано, что вирусная нагрузка варьирует от 105 до 109 копий/мл при поступлении и затем снижается экспоненциально по мере появления в крови специфических IgM- и IgG-антител. После 11–13-го дня болезни (7–12-го дня лечения) РНК вируса ККГЛ в крови не выявлялась. Из 21 изученных больных 17 было из ЮАР, 3 – из Ирана, 1 – из Пакистана.
Исследование 42 больных КГЛ из Косово (2001–2007 гг.) [31] показало, что вирусная нагрузка была максимальной в группе из 10 умерших пациентов (медиана – 3х109 копий/мл сыворотки крови, диапазон – от 6х107 до 1010 копий/мл), средней в группе из 15 пациентов с тяжелым течением болезни (медиана – 6х107, диапазон – от 3х104 до 9х108 копий/мл) и минимальной в группе из 17 пациентов со среднетяжелым течением (медиана – 2х105, диапазон – от 0 до 6х107 копий/мл). При этом вирусная нагрузка была выше 109 копий/мл у 8 из 10 умерших больных и ниже этого значения у всех выздоровевших. Измерения производили однократно для каждого пациента на 2–12-й день болезни (в среднем на 5–6-й день). Вирусная нагрузка отрицательно коррелировала с уровнями специфических IgM и IgG в крови больных (коэффициент корреляции Спирмена = 0,37).
Таким образом, зарубежные исследования подтвердили наши результаты не только качественно, но и количественно. Принципиально важно, что, как показывает сопоставление публикаций [26, 27, 29–31], сходные величины вирусной нагрузки у больных КГЛ наблюдаются в различных эпидемиологических условиях, при разных подходах к терапии и, вероятно, вне зависимости от генотипа вируса ККГЛ, вызвавшего заболевание. Однако патогенетическая роль высокой концентрации вируса ККГЛ в крови и других тканях должна быть опосредована иными биохимическими и цитологическими механизмами, отвечающими за повреждение клеток и органов-мишеней. В первую очередь в качестве таких эффекторов патогенеза рассматривались известные маркеры и медиаторы воспалительного процесса – цитокины.
Уровни ФНО-α, IL-10, ИФН-γ в крови больных коррелируют с вирусной нагрузкой (коэффициенты корреляции в диапазоне 0,3–0,6) и между собой (коэффициенты корреляции в диапазоне 0,3–0,8). У пациентов с тяжелым течением болезни они выше, чем у больных со среднетяжелым течением, но эти различия находятся на границах достоверности. Концентрации ФНО-α, IL-10 и ИФН-γ достоверно выше у умерших больных КГЛ по сравнению с выздоровевшими (приведены медианные значения, в скобках – диапазон): ФНО-α – 22 пг/мл (7–43) и 2,6 пг/мл (0–38); IL-10 – 220 пг/мл (42–490) и 41 пг/мл (1–210); ИФН-γ –28 пг/мл (6–490) и 9 пг/мл (0–91). Концентрация IL-12 не зависела от тяжести и исхода болезни и равнялась в среднем 26 пг/мл (диапазон от 0 до 155), то есть была ниже, чем у здоровых доноров. Вычисления проведены нами по табл. 1 из работы A. Saksida и соавт. [31], все обсуждаемые эффекты и отличия статистически значимы. Эти данные указывают на разбалансировку цитокиновой системы, возможно обусловленную высокой вирусной нагрузкой: секреция провоспалительных медиаторов ФНО-α и ИФН-γ может приводить к повреждению органов и тканей, в то время как высокий уровень регуляторного противовоспалительного цитокина IL-10 и низкий уровень IL-12 (индуктора клеточного иммунитета) могут свидетельствовать о нарушении иммунного ответа, что способствует дальнейшему размножению вируса КГЛ.
Согласно исследованиям, проведенным в Турции, в 1-ю неделю лечения в группе умерших пациентов по сравнению с группой вылечившихся была выше концентрация ФНО-α (10–75 и 4–41 пг/мл соответственно) и IL-6 (120–500 и 3–180 пг/мл соответственно). Уровень IL-10 не различался между группами и находился в диапазоне от 1 до 270 пг/мл [32]. В России концентрация цитокинов была изучена в динамике в 1-ю неделю у 38 больных КГЛ с благоприятным исходом заболевании. Отдельно были рассмотрены группы с тяжелым и среднетяжелым течением болезни, с геморрагическим синдромом и без него [33]. Уровень ФНО-α при КГЛ был в 4–5 раз выше нормы, уровень IL-2 – в 8–10 раз, а уровень противовоспалительного IL-4 – в 12–17 раз. Все эти показатели практически не зависели от клинического варианта заболевания. Напротив, концентрации IL-6 и IL-1β не только превышали норму в 5–15 раз, но и отражали особенности заболевания: IL-6 был в 2 раза выше при тяжелом течении болезни по сравнению со среднетяжелым; IL-1β был в 2–3 раза выше при КГЛ с геморрагическим синдромом и/или с тяжелым течением. В целом повышенные концентрации провоспалительных цитокинов не являются чем-то специфическим для КГЛ, но отражают развитие синдрома системного воспалительного ответа, характерного для многих вирусных и бактериальных заболеваний. Влияние системы цитокинов на тропизм вирусов на клеточном, органном и видовом уровне привлекает в последнее время повышенное внимание исследователей [34], но причины, по которым вирус ККГЛ вызывает не только лихорадку, но и повреждение стенок сосудов и другие гематологические нарушения, окончательно не ясны, что затрудняет эффективное лечение заболевания.
Лечение КГЛ
Патогенетическая терапия включает дезинтоксикационные, сердечно-сосудистые, противошоковые средства; при необходимости и под контролем показателей гемостаза – переливание донорской крови, плазмы, тромбоцитарной и/или эритроцитарной массы, кровезамещающих жидкостей с целью коррекции гемодинамических и гематологических расстройств и предотвращения профузных кровотечений [35–37].
В комплексной терапии КГЛ возможности этиотропного лечения крайне ограничены. Не получили развития попытки пассивной иммунотерапии КГЛ сывороткой крови иммунизированных лошадей или переболевших пациентов; терапия КГЛ специфическими поли- и моноклональными антителами не разработана [35, 38, 39]. Нет данных о клинической эффективности при КГЛ интерферонов или стимуляторов интерферонов, а также биоактивных молекул, регулирующих интерферон-зависимые реакции. Следует иметь в виду, что провоспалительный эффект ИФН-α может осложнить течение заболевания в активной фазе. Возможно, его рациональнее использовать для профилактики КГЛ или на самых ранних стадиях инфекции [19].
Такие элементы патогенеза КГЛ и других геморрагических лихорадок, как ДВС-синдром, гиперактивация эндотелия, дисрегуляция выработки цитокинов являются также важными элементами эндотоксинового (септического) шока [10], патогенез которого при бактериальных инфекциях, в частности при фульминантной менингококкцемии, хорошо изучен [40]. Поскольку летальность при эндотоксическом шоке является серьезнейшей проблемой и для развитых стран, ведущих интенсивные исследования в этой области, можно надеяться, что высокотехнологичные разработки методик и препаратов для борьбы с эндотоксическим шоком удастся впоследствии применить и при терапии КГЛ.
Активно обсуждается и достаточно широко исследуется возможность использования рибавирина – синтетического пуринового нуклеозида, выпускающегося как медицинский препарат под несколькими торговыми марками. В настоящее время рибавирин (в сочетании с другими препаратами) рекомендован FDA (U.S. Food and Drug Administration, http://www.fda.gov) только для лечения хронического гепатита С. Имеются публикации по применению рибавирина для терапии лихорадки Ласса, ГЛПС, вызванной вирусом Хантаан, респираторно-синцитиальной инфекции, ТОРС и др. Применение рибавирина для этиотропной терапии КГЛ основано на его доказанной антивирусной активности in vitro и in vivo (в опытах на мышах и в культуре клеток) [23, 41]. Рибавирин, после его внутриклеточного фосфорилирования, препятствует кэппированию и элонгации вирусной мРНК и ингибирует синтез рибонуклеопротеина [42]. С учетом фармакокинетики рибавирина при КГЛ рекомендована следующая схема: 30 мг/кг (орально) – начальная доза, 15 мг/кг каждые 6 ч в течение 4 дней, затем 7,5 мг/кг каждые 8 ч в течение 6 дней. При внутривенном введении использовалась схема: 17 мг/кг – начальная доза, 17 мг/кг каждые 6 ч в течение 4 дней, затем 8 мг/кг каждые 8 ч в течение 6 дней [43]. Противопоказания и возможные побочные действия препарата, включая тератогенный и эмбриотоксический эффекты, известны и должны учитываться при назначении [44, 45]. В Турции в 2004–2007 гг. 383 больных КГЛ были пролечены рибавирином, хотя масштаб применения его в целом снижался: в 2004 г. препарат получали 68% больных, в 2007 г. – только 12% [46].
Мета-анализ результатов 20 наиболее квалифицированных клинических исследований в Иране, Турции и Пакистане проведен в работе [47]. Во всех исследованиях, кроме одного [43], рибавирин применяли орально, в 10 исследованиях – в дозировке и по схеме ВОЗ, в остальных – по модифицированным схемам. В 12 исследованиях, включающих в сумме 955 больных, было зарегистрировано статистически значимое снижение летальности (на 44% от летальности в группах, не получавших рибавирин). Снижение можно было выявить только с помощью мета-анализа, поскольку по отдельности лишь в 3 публикациях [48–50] сообщается о статистически значимом влиянии препарата на летальность. Побочные эффекты рибавирина (анемия, тошнота, слабость и т.п.) наблюдались в большинстве исследований, но неизменно рассматривались как незначительные и не требующие прерывания лечения. Сокращения общей продолжительности лечения в результате применения рибавирина не выявлено. Хотя некоторые авторы полагают, что раннее (не позднее 5-го дня болезни) начало терапии рибавирином было бы более эффективным [50–52], мета-анализ имеющихся данных пока не подтверждает это предположение.
Используя методологию GRADE (Grading of Recommendations Assessment, Development and Evaluation) [53], авторы мета-анализа приходят к выводу, что имеющиеся свидетельства в пользу применения рибавирина должны быть расценены как «очень слабые». Более поздние проспективные исследования в Турции также не обнаружили влияния рибавирина на летальность, длительность госпитализации, вирусную нагрузку в крови больных КГЛ, восстановление количества тромбоцитов и лейкоцитов, нормализацию уровня АЛТ и АСТ [45, 54, 55]. Тем не менее, в ряде стран с высокой заболеваемостью КГЛ (Турция, Иран, Китай) врачи, опираясь на рекомендации ВОЗ, широко используют рибавирин и считают эту эмпирическую терапию КГЛ полезной для пациентов [39]. В этой ситуации некоторые специалисты по медицинской этике утверждают, что «было бы бесспорно неэтичным проводить рандомизированные плацебо-контролируемые исследования эффективности рибавирина для лечения этого жизнеугрожающего заболевания» [56], другие же исследователи настаивают на скорейшем проведении таких испытаний [45].
По данным исследования, проведенного в Астраханской области [57], включение рибавирина в комплексную терапию положительно влияет на течение КГЛ: сокращается продолжительность лихорадки и симптомов интоксикации, уменьшается интенсивность и длительность геморрагического синдрома. Следует отметить, что в этом исследовании как в группе больных, леченных рибавирином, так и в контрольной группе преобладали больные со среднетяжелым течением болезни, летальность отсутствовала. Наиболее систематически проанализированы результаты применения рибавирина для терапии КГЛ в Ставропольском крае [37]. Из 158 больных, получавших только базисную терапию (группа КГЛВ), умерли 18 (летальность 11%). В случае применения рибавирина по разработанной ставропольскими специалистами схеме летальность составила лишь 1,5% (4 из 268 пациентов, объединенных в группу КГЛР). Группы КГЛР и КГЛВ были сопоставимы по полу и возрасту больных и по срокам их госпитализации в инфекционный стационар. К сожалению, поскольку это исследование не являлось ни плацебо-контролируемым, ни двойным слепым, ни рандомизированным, его результаты могут подвергаться сомнению с позиций доказательной медицины. Отсутствие плацебо вряд ли является серьезным недостатком при изучении такого тяжелого заболевания, как КГЛ. То, что врачи знали, к какой группе относится больной, могло в некоторой степени повлиять на ведение и обследование больных, назначение лабораторных анализов, интерпретацию наблюдений. Принципиальной проблемой является отсутствие рандомизации. Около 85% больных, включенных в группу КГЛВ, заболели в период от 1999 до 2002 г., когда летальность от КГЛ на Ставрополье составляла 8%. При этом не сформулированы критерии, по которым ряду тяжелых (впоследствии умерших) больных КГЛ, включенных в группу КГЛВ в 2003–2008 гг., не назначали рибавирин. Все больные из группы КГЛР лечились им в 2003–2008 гг. Поэтому допустимо объяснение, что снижение летальности после 2002 г. происходило по мере накопления опыта и повышения качества базисной терапии КГЛ. Весьма вероятно и подтверждается данными, приведенными в работе [37], что в 2003–2008 гг., на фоне повышенной настороженности и улучшенной диагностики, в группу КГЛР просто было включено больше больных с легким и среднетяжелым течением заболевания (90%) и без геморрагического синдрома (60%). В группе КГЛВ эти величины составили соответственно 69 и 17%. Если сравнить летальность только по подгруппам больных с тяжелым течением болезни, она составит 14% в группе КГЛР и 36% в группе КГЛВ. Разница уже не столь высока и находится на границах статистической достоверности (p = 0 ,04, точный критерий Фишера). С другой стороны, возможно само увеличение доли больных КГЛ с легким и среднетяжелым течением заболевания и без геморрагического синдрома обусловлено широким применением рибавирина, начиная с 2003 г. В попытке преодолеть эту трудность авторы используют понятие больные «с предикторами летального исхода КГЛ», сформулированными ранее [37, 58]. Среди больных, леченных рибавирином, таких обнаруживается всего 8 человек. В результате летальность среди «больных с предикторами летального исхода КГЛ» не различается в группах КГЛР и КГЛВ, составляя около 50%. Следует признать, что хотя различие в летальности от КГЛ в 1999–2002 гг. и 2003–2008 гг. в Ставропольском крае весьма велико и статистически достоверно, его нельзя однозначно отнести к эффекту применения рибавирина.
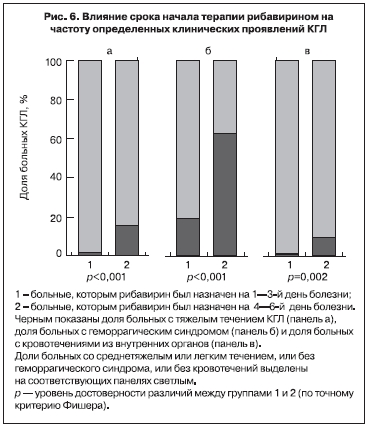
Однако работа [37] содержит и другие важные наблюдения. 260 больных без «предикторов летального исхода КГЛ» из группы КГЛР можно разбить на две приблизительно равные подгруппы: 142 пациента, которым рибавирин был назначен уже на 1–3-й день болезни, и 118 пациентов, у которых терапия рибавирином была начата в поздние сроки, от 4-го до 6-го дня болезни. Оказалось, что доля тяжелых форм заболевания, доля заболеваний с геморрагическим синдромом и доля пациентов с кровотечениями из внутренних органов были существенно ниже в подгруппе больных КГЛ, получавших терапию своевременно (рис. 6). Это явление, наблюдавшееся у больных, лечившихся в одни и те же годы (с 2003 по 2008 г.), позволяет высказать гипотезу, что раннее начало терапии рибавирином частично блокирует прогресс заболевания от среднетяжелого состояния без геморрагий к тяжелому, с геморрагическим синдромом. Изучение динамики концентраций лейкоцитов и тромбоцитов в крови показало, что они, вне зависимости от применения рибавирина, снижались до минимальных значений к 4–6-му дню болезни. Однако в группе больных, получавших рибавирин, восстановление нормального уровня лейкоцитов и тромбоцитов происходило существенно быстрее. Также в группе, получавшей рибавирин, наблюдалось лишь незначительное увеличение АЧТВ в начале болезни, проходящее к концу 1-й недели, а у больных КГЛ, получавших только базисную терапию, величина АЧТВ превосходила норму в 1,5–2 раза и нормализовалась не раньше 11-го дня болезни.
В целом анализ отечественного опыта позволяет присоединиться к мнению ряда российских и зарубежных специалистов: применение рибавирина в ранние сроки от начала заболевания может способствовать снижению числа тяжелых и, возможно, даже летальных форм КГЛ и является на настоящем этапе элементом оптимальной терапии КГЛ. Однако дискуссии последнего десятилетия показали, что стать общепринятым это мнение может только в том случае, если будет подтверждено результатами рандомизированных двойных слепых плацебо-контролируемых исследований.
Представляются перспективными, но находятся пока на самых начальных стадиях разработки методы «нового поколения» – специфическая терапия найровирусных инфекций, использующая феномен РНК-интерференции для блокирования синтеза нуклеопротеина, и «антивирусная терапия широкого спектра», провоцирующая апоптоз клеток, содержащих двухцепочечную вирусную РНК [59, 60].
В первом случае авторы синтезировали и испытали так называемые «малые интерферирующие РНК» (siRNA), способные ингибировать размножение найровируса Хазара в культуре клеток альвеолярного эпителия человека А549. Наиболее эффективными оказались препараты siRNA, взаимодействующие с сегментом S, кодирующим нуклеопротеин: репликация вируса уменьшилась на 88–92%. Отобранные препараты siRNA в концентрации 100 нМ были не токсичны для клеток человека. При этом они проявляли выраженную антивирусную активность, если проникали в клетки в интервале от 3 сут до заражения вирусом до 1 сут после него. Была показана синергия двух веществ с разными принципами действия – рибавирина и siRNA: эффект смеси препаратов был сильнее, чем сумма эффектов каждого из них в отдельности [59].
Авторы второй работы создали несколько химерных молекул, которые с одного конца имеют домен, отвечающий за распознавание вирусной двухцепочечной РНК (фрагмент зависимой от двухцепочечной РНК киназы PKR или РНК-азы RNaseL), а с другого конца – домен, активирующий апоптоз [фрагмент «фактора, активирующего апоптоз» (Apaf-1), или домен «активированной смерти» (FADD)]. Получившиеся препараты, названные DRACO, проникали в культивируемые клетки и, как было показано для клеток различного происхождения (человеческих, обезьяньих, мышиных) и из различных органов (легких, печени, сердца, почек и т. д.), были для них сами по себе не токсичны. Однако, если те же самые клетки были инфицированы вирусом, препарат активировал апоптоз зараженных клеток. В результате вирус полностью элиминировался из культуры через несколько суток, а оставшиеся незараженными клетки продолжали успешно размножаться. Принципиально, что препарат DRACO был эффективен против 15 видов вирусов из различных семейств, имеющих различные структуры вириона и генома (одноцепочечная РНК положительной или отрицательной полярности, двухцепочечная РНК), распознающих различные рецепторы на поверхности клетки, размножающихся в цитоплазме или ядре, вызывающих заболевание человека или грызунов. В частности, препарат DRACO был эффективен в культуре клеток против таких имеющих высокое эпидемическое значение вирусов, как вирус гриппа H1N1 и вирус денге, а также, что интересно в контексте этого обзора, против буньявируса Гуама и аренавирусов. Более того, было показано, что DRACO защищает мышей от летальных доз вируса гриппа H1N1. Таким образом, препараты типа DRACO потенциально могут быть использованы для профилактики или терапии различных острых вирусных инфекций [60].
Таким образом, можно констатировать, что в XXI веке, отчасти из-за ухудшения эпидемиологической обстановки по КГЛ, исследования патогенеза этого заболевания и подходов к терапии КГЛ были резко интенсифицированы. Яснее стали механизмы взаимодействия вируса ККГЛ с клетками в культуре, появляются перспективные лабораторные модели инфекции на иммунодефицитных животных. Ведутся активные испытания доступных противовирусных препаратов, регламентируется в виде методических документов комплекс мероприятий по патогенетической и восстановительной терапии. Все это позволяет надеяться на продолжение и усиление наметившейся тенденции к снижению летальности КГЛ. Особенно обнадеживающим выглядит развитие науки и биотехнологии, обещающее в недалеком будущем появление принципиально новых и эффективных противовирусных средств и методов терапии.


