COVID-19 представляет собой высококонтагиозную патогенную вирусную инфекцию, вызванную штаммом SARS-CoV-2, быстро распространившуюся по всему миру. В первую очередь новая коронавирусная инфекция поражает дыхательную систему, но появляется все больше данных о вовлечении в патогенез инфекции желудочно-кишечного тракта [1]. Помимо наиболее распространенных симптомов COVID-19 – лихорадки, кашля и миалгии, наблюдались также боль в горле, головная боль, озноб, тошнота, рвота и диарея. Желудочно-кишечные симптомы, которые сохранялись в течение длительного времени, включали тошноту, боль в животе, потерю аппетита, изжогу и запор [2–4]. Кишечные расстройства, наблюдаемые при тяжелом течении инфекции SARS-CoV-2, связаны как с повреждающим действием энтероцитов путем связывания с ангиотензинпревращающим рецептором 2 (ACE2) [5], так и с нарушением микробиоценоза кишечника. Существуют данные, что состав кишечной микрофлоры значительно изменяется у пациентов с COVID-19, снижается колонизационная резистентность и нарушается баланс в структуре филометаболического ядра [6, 7].
Микробиоценоз кишечника имеет важное общебиологическое значение для организма человека на протяжении всей его жизни [8]. Облигатная микрофлора кишечника участвует в регулировке физиологических, биохимических и иммунологических процессов в организме, в том числе в защите от патогенов, и поддерживает метаболизм питательных веществ [8, 9]. Фактически желудочно-кишечный тракт считается крупнейшим иммунным органом в организме, который может регулировать иммунитет хозяина, защищать от патогенов и поддерживать метаболизм питательных веществ [9]. Кишечный микробиоценоз играет большую роль в поддержании здоровья человека, в том числе в обеспечении защиты от кишечных бактериальных патогенов, но ряд факторов, таких как инфекции или необоснованное применение антибиотиков, могут нарушить этот баланс [10]. Сохраняется актуальность использования антибиотиков в период пандемии COVID-19.
Цель исследования – анализ влияния пандемии новой коронавирусной инфекции, вызванной вирусом SARS-CoV-2, на структуру микробиоценоза кишечника по сравнению с показателями микробиоценоза до пандемии.
Материалы и методы
Методом случайной выборки был проведен ретроспективный анализ результатов исследования микробиоценоза кишечника среди населения Российской Федерации до и во время пандемии COVID-19. Число пациентов в возрасте от 1 до 60 лет, обследованных в период с января 2018 г. по декабрь 2022 г. составило 72 798.
Исследовали пробы фекалий по мере их поступления в лаборатории лечебных учреждений. Для приготовления суспензии 0,5 г клинического материала, поступавшего в специальных контейнерах, эмульгировали в солевом буфере с редуцирующими компонентами. Затем готовили ряд последовательных десятикратных разведений материала на том же буфере и засевали на чашки Петри с питательными средами. Посев клинического материала на питательные среды и учет результатов проводили согласно ОСТ 91500.11.0004-2003 «Протокол ведения больных. Дисбактериоз кишечника» на следующие питательные среды: агар Эндо и Плоскирева для изоляции бактерий семейства Enterobacterales, желточно-солевой агар – для бактерий семейства Staphylococcaceae, энтерококковый агар – для культур рода Enterococcus spp., среда МРС с сорбиновой кислотой – для лактобактерий, Бифидум-среда – для бифидобактерий, среда Шедлера – для выделения основной группы анаэробных бактерий и 5% кровяной агар – для бактерий рода Streptococcus spp., Clostridium spp., а также для других видов микроорганизмов, нуждающихся для своего роста в крови или гемолизирующих ее. С целью повышения эффективности выделения клостридий использовали этаноловый шок [11]. Анаэробные бактерии культивировали в специальных системах типа «ГазПак» производства фирмы Oxoid (Thermo Fisher Scientific, США), работающих на принципе химической адсорбции кислорода. Идентификацию выделенных штаммов бактерий осуществляли традиционными методами, при необходимости использовали метод матрично-активированной лазерной ионизации-/времяпролетной масс-спектрометрии (MALDI-TOF MS) с применением системы Microflex LT и программного обеспечения MALDI Biotyper Compass v.4.1.80 (Bruker Daltonics, Германия). После определения вида микроорганизма производили пересчет количества его колоний на 1 г фекалий (КОЕ/г).
Статистическую обработку результатов проводили с использованием стандартных методов описательной статистики с помощью программы Microsoft Office Excel 2010. Статистическую значимость различий доли культур оценивали с помощью t-критерия Стьюдента при уровне значимости α < 0,05 (t > 2).
Результаты
До пандемии доля пациентов с нормальными показателями микробиоценоза желудочно-кишечного тракта среди обследованных в Центральном НИИ эпидемиологии Роспотребнадзора составляла от 97,6 ± 0,23 до 99,1 ± 0,10%. С марта 2020 г. частота обнаружения облигатных симбионтов кишечника снизилась с 67,3 ± 1,40 до 22,1 ± 3,59% (p < 0,01).
Распространение вируса SARS-CoV-2 среди населения коррелировало с ростом нарушений микробиоценоза кишечника с 0,9 ± 0,10% – 2,4 ± 0,23% (2018 – март 2020 г.) до 32,7 ± 1,40% – 77,9 ± 3,59% (апрель 2020 – 2022 г.) в зависимости от месяца изучения (p < 0,01).
Изучение показателей микробиоценоза во время пандемии COVID-19 показало, что пик нарушений со стороны микробиоценоза кишечника возникает через 2–3 мес. после пиковых значений заболеваемости новой коронавирусной инфекцией (см. рисунок).

Анализ состава микробиоценоза в 2018–2022 гг. выявил тенденцию к увеличению дефицита облигатной микрофлоры кишечника – бифидобактерий и бактерий рода Enterococcus spp. – с каждым новым подъемом заболеваемости, вызванным вирусом SARS-CoV-2 (табл. 1.). Снижение доли бифидобактерий составляло от 2,4 ± 0,9% в период циркуляции геноварианта B.1.1 до 34,1 ± 0,71% в период доминирования геноварианта BA.4.5. Также было выявлено достоверное снижение доли бактерий рода Enterococcus spp. с 15,0 ± 0,29% в период циркуляции геноварианта Alpha до 46,3 ± 0,88% в период доминирования геноварианта Delta (p < 0,01).
Максимальный дефицит лактобактерий, по сравнению с периодом до пандемии COVID-19, наблюдался во время преобладания геновариантов Alpha и B.A.4.5 – 54,2 ± 0,57% и 54,0 ± 0,79% соответственно; лактозопозитивных (типичных) E. coli – при доминировании геновариантов Delta (39,8 ± 0,85%) и Omicron (49,5 ± 0,8%) (p < 0,01) (см. табл. 1).
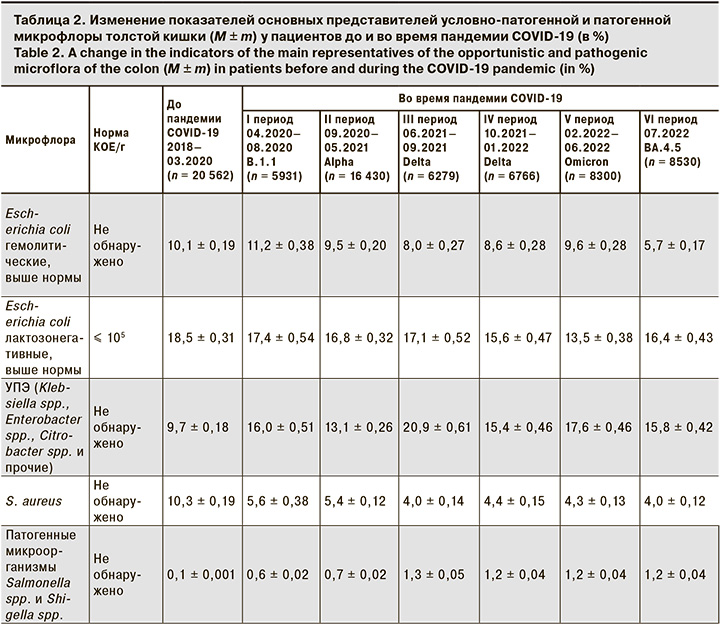
Анализ данных условно-патогенной флоры показал, что в период до пандемии COVID-19 превышение доли S. aureus наблюдалось в 10,3 ± 0,19% случаев, лактозонегативных и гемолитических форм кишечных палочек – 18,5 ± 0,31% и 10,1 ± 0,19% соответственно (табл. 2). Во время пандемии новой коронавирусной инфекции COVID-19 S. aureus и лактозонегативные формы кишечных палочек были выявлены у 5,6 ± 0,38% и 17,4 ± 0,54% пациентов соответственно. Выявляемость гемолитической формы E. coli в период первого подъема заболеваемости новой коронавирусной инфекцией выросла с 10,1 ± 0,19% до 11,2 ± 0,38% (p < 0,01), затем наблюдалось снижение до 9,5 ± 0,20% при преобладании геноварианта Omicron (p < 0,01).
Наиболее заметные отличия в структуре микробиоценоза были выявлены для представителей условно-патогенных энтеробактерий (УПЭ) – Klebsiella spp., Enterobacter spp., Citrobacter spp., Proteus spp. и Roultella spp., доля которых увеличилась с 9,7 ± 0,18% в 2018–03.2020 до 20,9 ± 0,61% во время доминирования геноварианта Delta. Достоверные различия были также выявлены для патогенных микроорганизмов Salmonella spp. и Shigella spp. До пандемии новой коронавирусной инфекции COVID-19 патогенные микроорганизмы выявлены у 0,1 ± 0,001% пациентов на фоне пандемии у 1,3 ± 0,05% (p < 0,01) (см. табл. 2).
Обсуждение
Новая коронавирусная инфекция SARS-CoV-2 распространилась по всему миру и оказала огромное влияние на глобальную политику и экономику. В настоящее время пандемия COVID-19 полностью не контролируется и неоднократно повторялась. Согласно данным литературы, с начала пандемии COVID-19 в России был отмечен рост потребления антибиотиков на 13,5% [12]. В период эпидемического роста заболеваемости в 2020–2021 гг. самая высокая частота потребления противомикробных препаратов отмечена для макролидов, фторхинолонов и комбинации пенициллинов с ингибиторами бета-лактамаз, такими как амоксициллин с клавулановой кислотой [12].
В наших исследованиях максимальный дефицит облигатной симбиотической микрофлоры приходится на июль–август 2021 г. и август–сентябрь 2022 г., которые соответствуют периодам циркуляции геновариантов Delta и Omicron SARS-CoV-2, что согласуется с научными исследованиями по распределению геновариантов вируса SARS-CoV-2 по периодам эпидемического роста заболеваемости COVID-19 на территории России за 2020–2022 гг. [13]. Снижению выявленной нами колонизационной резистентности кишечника могло способствовать излишне частое потребление антибиотических препаратов во время доминирования геновариантов В.1.1 в период 04–08.2020 и Alpha в период 09.2020–05.2021, что привело к медленному и неполному восстановлению микробиоценоза на фоне повреждающего воздействия самого вируса. Исследования J. Gang и соавт. [14] показали, что инфекция SARS-CoV-2 оказывает неблагоприятное воздействие на респираторную, кишечную и ротовую микробиоту организма, проявляющееся прежде всего в снижении микробного разнообразия и однородности, уменьшении количества полезных симбиотических бактерий.
Нарушение кишечного микробиоценоза является предрасполагающим фактором для провоспалительных состояний (например, сепсиса), хотя механизм изменения кишечного микробиоценоза, лежащий в основе тяжелых состояний, до сих пор неясен. Предположительно дисбиоз кишечника человека может быть одним из возможных факторов, лежащих в основе тяжелого течения COVID-19. Данная гипотеза требует дополнительных сравнительных исследований тяжести COVID-19, включая фазу выздоровления COVID-19, фазу выработки антител против SARS-CoV-2 и кишечной микрофлоры [10]. Многочисленные исследования говорят о том, что симптомы бактериального дисбиоза кишечника могут сохраняться даже после элиминации вируса [14, 15].
Анализ состояния микробиоценоза изученных нами пациентов в период циркуляции вируса SARS-CoV-2 выявил, что симптомы бактериального дисбиоза вызваны превалированием условно-патогенной флоры Klebsiella spp., Enterobacter spp., Citrobacter spp. и др. В исследованиях S. Yamamoto и соавт. [7] были получены аналогичные результаты: у пациентов с COVID-19 наблюдался дисбиоз кишечника с повышением количества условно-патогенной флоры Clostridium hathewayi, Actinomyces viscosus, Morganella morganii [7]. Также нами выявлен рост патогенной флоры (Salmonella spp. и Shigella spp.) на фоне снижения уровней Lactobacillus spp. и Bifidobacterium spp., что согласуется с исследованием других авторов, где показано, что у пациентов с COVID-19 на фоне снижения уровней Lactobacillus spp. и Bifidobacterium spp. увеличивается количество патогенных бактерий в кишечнике, включая Corynebacterium spp. и Ruthenibacterium spp. [7]. И наоборот, образцы фекалий с низкой активностью SARS-CoV-2 или без нее имели повышенный уровень бактерий, принадлежащих к Parabacteroides spp., Bacteroides spp. и Lachnospiraceae spp., которые продуцируют короткоцепочечные жирные кислоты, играющие важную роль в повышении иммунитета человека [7]. Эти данные свидетельствуют о том, что нарушение микробиоценоза как представляет угрозу для снижения иммунитета человека, так и способствует росту инфекций, вызванных условно-патогенной и патогенной флорой, пропорционально росту заболеваемости вирусом SARS-CoV-2.
Заключение
Результаты проведенного нами исследования, а также данные литературы свидетельствуют о том, что в период пандемии новой коронавирусной инфекции SARS-CoV-2 у населения РФ в возрасте от 1 года до 60 лет отмечены достоверно значимые изменения микробиоценоза кишечника, заключающиеся в снижении облигатных симбионтов бифидобактерий, лактобактерий, энтерококков и лактозопозитивных (типичных) E. coli, повреждении филометаболического ядра микробиоценоза кишечника с преобладанием условно-патогенной и патогенной флоры. Факторами, влияющими на микробиоценоз, могли быть как действие самого вируса SARS-CoV-2, так и нерациональная антибиотикотерапия.


