Появление и внедрение антиретровирусной терапии (АРТ) в практику оказания помощи людям, живущим с ВИЧ, позволило перевести заболевание в хроническую форму [1]. Продолжительность жизни таких пациентов с высокой приверженностью терапии не отличается от продолжительности жизни людей, живущих без ВИЧ [2]. Но на сегодняшний день полностью излечиться от ВИЧ-инфекции невозможно, и даже при многолетней эффективной терапии полного уничтожения вируса в организме достичь не удается. Причиной этого является существование резервуара ВИЧ [3] – клеток, инфицированных репликативно-компетентным вирусом в разных тканях и анатомических зонах. Такие клетки присутствуют в организме человека, несмотря на длительный прием антиретровирусных препаратов (АРВП) [4]. После прерывания терапии через определенное время вирус, находящийся в латентном состоянии, возобновляет репликацию из-за исчезновения лекарственного подавления, и ВН возрастает [3]. Таким образом, существование резервуара – основная причина необходимости пожизненного регулярного приема АРВП.
Исследования показали, что размер резервуара влияет на множество параметров, таких как скорость прогрессирования болезни [5], риск развития ВИЧ-ассоциированных заболеваний [6, 7], время достижения недетектируемой ВН при приеме АРВП [8]. С. Rouzioux и соавт. [9] установили, что концентрация ДНК ВИЧ в мононуклеарных клетках периферической крови (МКПК) является основным предиктором прогрессирования заболевания, снижения количества CD4+-лимфоцитов до уровня < 200 клеток/мкл и наступления СПИДа, независимо ни от концентрации РНК ВИЧ, ни от количества CD4+-лимфоцитов.
ДНК ВИЧ в организме человека может существовать в разных формах: интегрированной в хромосому клетки (интактный и дефектный провирус), и неинтегрированной (кольцевые длинные концевые повторы 1-LTR, 2-LTR и линейная ДНК) [10]. Все эти формы суммарно называются тотальной ДНК ВИЧ.
Для каждой формы существуют свои методы оценки. До недавнего времени «золотым стандартом» измерения вирусного резервуара считался QVOA (Quantitative Viral Outgrowth Assay) [11], но в результате дальнейших исследований стало ясно, что этот метод недооценивает фактический размер вирусного резервуара, потому как не все CD4+-лимфоциты пролиферируют после первого раунда активации. К тому же этот метод оказался дорогим, трудоемким, длительным и неприменимым для клинических исследований. На смену ему пришли методы на основе ПЦР, такие как Alu-PCR [12], IPDA [13] и Q4PCR [14], нацеленные на несколько областей провирусной ДНК, тем самым исключая из анализа дефектные провирусы, содержащие гипермутации, вставки, делеции и др. Коллектив BEAT-HIV Martin Delaney Collaboratory выпускает рекомендации, отдавая приоритет IPDA или Q4PCR для измерения размера резервуара ВИЧ в крови и тканях во время клинических испытаний, связанных с лечением ВИЧ [4]. Эти методы позиционируются как дешевые, быстрые и простые по сравнению с QVOA, позволяющие оценить уровень репликативно-компетентного вируса. Из недостатков исследователи выделяют высокую вариабельность от пациента к пациенту, так как в некоторых случаях наблюдалась значительная частота ложного обнаружения, дефектные провирусы признавались за интактные либо не давали сигнала. Объясняется это полиморфизмом последовательностей в провирусных геномах и требованием гибридизации с третьим и/или четвертым зондом, что снижает чувствительность этих методов [4].
Дефектный провирус может быть трансляционно-активным и «мертвым», то есть неспособным продуцировать вирусные белки [15]. Ввиду этого такой маркер, как тотальная ДНК ВИЧ подвергается критике из-за невозможности дифференциации интегрированной и неинтегрированной форм, а также интактного и дефектного провируса [16], который составляет около 98% от всей ДНК ВИЧ в организме [17]. Однако это можно считать и преимуществом, поскольку дефектные провирусы вносят свой вклад в патогенез заболевания, играют роль в репликации и патофизиологии: продуцируя вирусные компоненты (gp120, gp41, Tat, Rev, Nef, Vpr и Vpu), они вызывают иммунный ответ и гиперактивацию иммунитета, что способствует распространению ВИЧ по организму [16]. H. Imamichi и соавт. [18] показали, что «дефектные» провирусы способны продуцировать разные виды несплайсированной РНК ВИЧ у пациентов с подавленной ВН в течение более 6 лет. Ученые полагают, что стойкие «дефектные» провирусы способны стимулировать пути защиты, нацеленные на чужеродные для организма белки и нуклеиновые кислоты.
В качестве маркера для исследования резервуара нами была выбрана тотальная ДНК ВИЧ. Анализ этого маркера с помощью ПЦР в реальном времени является быстрым, простым в выполнении, воспроизводимым, точным и чувствительным, позволяя охарактеризовать глобальный размер резервуара ВИЧ.
На текущий момент измерение размера резервуара ВИЧ является актуальным направлением, поскольку большое число исследований проводится в области разработки новых терапевтических подходов к лечению и полной эрадикации ВИЧ из организма [19, 20].
В связи с этим целью настоящего исследования являлась разработка метода определения размера резервуара, измерение его у людей, живущих с ВИЧ (ЛЖВ) без опыта приема АРТ и анализ его связи с ВН, длительностью инфекции и иммунным статусом.
Материалы и методы
В исследовании были использованы МКПК от 268 ВИЧ-инфицированных пациентов с известными сроками заражения, установленными по результатам эпидрасследования и ИФА, определения p24-антигена и иммунного блоттинга. Все пациенты не имели опыта приема АРВП. Длительность инфекции на момент забора крови варьировала от 0,9 до 92,9 мес. (медиана – 7,6 мес.) и была определена как разница между датой предполагаемого инфицирования и датой забора крови для оценки размера резервуара. У пациентов определяли показатель ВН и иммунный статус. ВН была известна у всех пациентов, иммунный статус – у 185 из 268 (69%).
Возраст пациентов варьировал от 13 лет до 71 года; мужчины составляли 57,1%, женщины – 42,9%.
У 258 (96,3%) пациентов, включенных в выборку, был известен путь заражения. Основным путем заражения был гетеросексуальный – 74,8%. Гомосексуальным, инъекционным и нозокомиальным путем заразились 17,1, 7,4 и 0,8% пациентов соответственно.
Кровь забирали в вакуумные пробирки типа Vacuette (с EDTA), центрифугировали (2000 об/мин, 20 мин), затем отбирали 0,25 мл лейкоцитарного кольца, располагающегося между плазмой и эритроцитами, которое затем дважды отмывали гемолитиком. Полученный осадок клеток использовали для экстракции ДНК при помощи набора реагентов РИБО-преп (Центральный НИИ эпидемиологии Роспотребнадзора, далее – ЦНИИЭ).
Для проведения ПЦР с детекцией в режиме реального времени использовали набор реагентов АмплиСенс® ДНК-ВИЧ-FL (ЦНИИЭ). Чтобы определить концентрацию ДНК ВИЧ в образцах, в каждой постановке использовали высококопийный и низкокопийный калибраторы с известной концентрацией в 2 повторах каждый, которые представляли собой разведение плазмиды со встроенным фрагментом гена pol ВИЧ. Параллельно для измерения числа клеток, попавших в реакцию, в каждой постановке участвовали высококопийный и низкокопийный калибраторы с известной концентрацией в 2 повторах каждый, которые представляли собой разведение плазмиды со встроенным фрагментом гена β-глобина человека, а в ПЦР смеси присутствовали праймеры на эту область.
Число копий ДНК ВИЧ/106 МКПК рассчитывали по формуле:

Окончательные результаты выражали в log10 копий ДНК ВИЧ/106 МКПК.
Статистическую обработку данных проводили с помощью программного обеспечения SPSS Statistics. Для оценки связи между вирусологическими маркерами и другими переменными использовали коэффициент корреляции Пирсона (r), для сравнения различных показателей – U-критерий Манна–Уитни. Достоверными считали различия при p < 0,05.
Результаты
На основании полученных в ПЦР значений порогового цикла – Ct (рис. 1, а, б) и, исходя из известных значений высококопийного и низкокопийного калибраторов со встроенным фрагментом гена ВИЧ и высококопийного и низкокопийного калибраторов со встроенным фрагментом гена β-глобина человека, строятся калибровочные графики (рис. 1, в, г).

Полученные в ПЦР значения Ct для исследуемых образцов используются для расчета числа копий ВИЧ и числа копий фрагмента гена β-глобина человека, попавших в реакцию.
Концентрация ДНК ВИЧ, измеренная нашим методом, варьировала от 0,04 до 3,84 log10 копий ДНК ВИЧ/106 МКПК (медиана – 2,22 log10 копий ДНК ВИЧ/106 МКПК, межквартильный диапазон – 1,74–2,72 log10 копий ДНК ВИЧ/106 МКПК). Показатели ВН и иммунного статуса составляли от 1,28 до 7,08 log10 копий РНК/мл (медиана – 4,68 log10 копий РНК/мл) и от 23 до 1054 клеток/мкл (медиана – 459 клеток/мкл) соответственно.
Полученные в ПЦР значения Ct для исследуемых образцов используются для расчета числа копий ВИЧ и числа копий фрагмента гена β-глобина человека, попавших в реакцию.
Концентрация ДНК ВИЧ, измеренная нашим методом, варьировала от 0,04 до 3,84 log10 копий ДНК ВИЧ/106 МКПК (медиана – 2,22 log10 копий ДНК ВИЧ/106 МКПК, межквартильный диапазон – 1,74– 2,72 log10 копий ДНК ВИЧ/106 МКПК). Показатели ВН и иммунного статуса составляли от 1,28 до 7,08 log10 копий РНК/мл (медиана – 4,68 log10 копий РНК/мл) и от 23 до 1054 клеток/мкл (медиана – 459 клеток/мкл) соответственно.
Был проведен анализ наличия ассоциаций между размером вирусного резервуара и различными показателями пациентов. Концентрация ДНК ВИЧ коррелировала с ВН (r = 0,526; p < 0,01) и имела слабую обратную корреляцию с показателем иммунного статуса (r = -0,189; p < 0,05) (рис. 2, а, б).
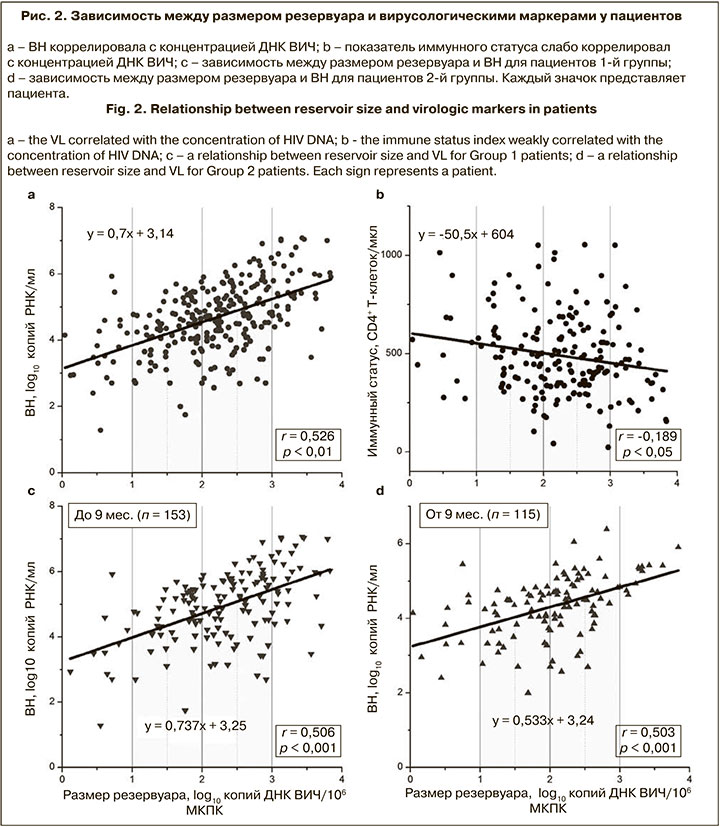
В зависимости от длительности ВИЧ-инфекции пациенты были разделены на 2 группы: в 1-ю группу (п = 153) вошли пациенты с длительностью инфекции до 9 мес., во 2-ю (п = 115) – от 9 мес. Графики зависимости ВН от размера резервуара для этих групп представлены на рис. 2, в, г.
Установлено, что медианные значения концентрации ДНК ВИЧ статистически значимо различались и составляли 2,32 и 2,08 log10 копий ДНК ВИЧ/106 МКПК для 1-й и 2-й групп соответственно (p < 0,01).
Для пациентов с известным показателем иммунного статуса был построен график зависимости между размером резервуара и ВН (рис. 3). У 48 из 185 пациентов (26%) он был < 350 клеток/мкл, у остальных 137 (74%) – > 350 клеток/мкл.

Концентрация ДНК ВИЧ у пациентов с количеством CD4+-лимфоцитов > 350 клеток/мкл и < 350 клеток/мкл не отличалась (p = 0,207).
Размер резервуара отрицательно коррелировал с длительностью инфекции (r = -0,282; p < 0,01). Диапазон размера резервуара у пациентов представлен на рис. 4.
Обсуждение
У участников исследования мы наблюдали широкий диапазон концентрации ДНК ВИЧ. При этом размер вирусного резервуара у пациентов без опыта приема АРТ связан с вирусологическими показателями. Корреляция между концентрацией ДНК, измеренной приведенным выше методом, и ВН, а также между концентрацией ДНК и показателем иммунного статуса согласуется с результатами, опубликованными ранее [9, 21–23].
Хотя количество CD4+-лимфоцитов уменьшается с течением инфекции, для пациентов с показателем иммунного статуса > 350 и < 350 клеток/мкл нет различий в концентрации ДНК ВИЧ. Предположительно, это связано с индивидуальностью естественного течения болезни, а также с тем, что при снижении количества CD4+-лимфоцитов неизбежно снижается вероятность обнаружения ДНК ВИЧ.
Концентрация ДНК ВИЧ у пациентов снижалась с течением инфекции, и поскольку со временем количество инфицированных клеток уменьшается, цель исследования состоит в том, чтобы количественно оценить это редкое событие. Такая количественная оценка соответствует распределению вероятностей Пуассона. Применяя любой метод, необходимо тестировать большое количество клеток, чтобы достичь низких пределов обнаружения.
Не отмечено различий в связи концентрации ДНК ВИЧ с ВН у пациентов с ранней и поздней инфекцией, однако медианное значение размера резервуара оказалось статистически значимо выше для пациентов с длительностью инфекции до 9 мес. Данные ранее опубликованного метаанализа [24], включающего 1047 образцов, свидетельствуют, что концентрация ДНК ВИЧ является сильным прогностическим маркером прогрессирования ВИЧ-инфекции до стадии СПИДа. C. Goujard, и соавт. [25] установили, что концентрация тотальной ДНК ВИЧ в первые 6 мес. после сероконверсии позволяет прогнозировать прогрессирование нелеченной инфекции. В связи с этим необходимо стремиться использовать этот маркер уже на ранних этапах диагностики.
Заключение
Результаты проведенного исследования показывают, что концентрация ДНК ВИЧ предоставляет полезную дополнительную информацию для каждого пациента, этот маркер может дополнять показатель ВН. Однако его не следует рассматривать как замену концентрации РНК ВИЧ в плазме, которая остается важной для оценки эффективности АРТ. Концентрация ДНК ВИЧ может предоставить дополнительную информацию во время острой инфекции или первого положительного результата теста на ВИЧ. Учитывая наличие побочных эффектов при долгосрочном приеме АРВП, размер резервуара может являться фактором, учитывающимся при принятии решения об упрощении схемы АРТ, а также позволяет оценить ее эффективность при недетектируемой ВН в долгосрочной перспективе. Представленный нами метод определения концентрации ДНК ВИЧ может быть использован в клинических исследованиях по разработке новых терапевтических подходов и полной эрадикации ВИЧ.


