Заболевание, вызванное вирусом Varicella zoster (VZV), известно с давних времен и по-прежнему имеет широкое распространение в современном мире. Благодаря вакцинопрофилактике в последнее время снижается бремя заболеваемости по большинству инфекций вирусной этиологии с аэрогенным механизмом передачи (корь, паротит, краснуха, грипп). На этом фоне значительно возрастает роль инфекций, вызванных VZV: ежегодно регистррируется более 4,2 млн случаев заболеваний, требующих госпитализации в связи с осложнениями, и 4200 смертельных исходов [1–5].
Этиологическим агентом является высоко нейротропный герпесвирус, который способен размножаться во многих органах и тканях человека [3–5]. VZV имеет 5 генетических вариантов, различия между ними варьируют в пределах 0,2%. Вирус малоустойчив к внешним воздействиям, термолабилен и чувствителен к дезинфекционным средствам, однако может длительное время сохраняться в условиях низких температур. Считается, что VZV может вызывать 2 формы заболевания: ветряную оспу (ВО), протекающую преимущественно в виде экзантемы, и опоясывающий лишай, развивающийся в результате реактивации вируса, сохраняющегося в латентной форме в дорсальных сенсорных ганглиях [5, 6].
ВО широко распространена в популяции людей и чаще всего ассоциируется с легким заболеванием, возникающим в детском возрасте. По данным ВОЗ, около 90% детей, живущих в странах с умеренным климатом, переносят инфекцию, вызванную VZV, к 15 годам. В Российской Федерации в допандемический период ежегодно регистрировалось 700 000–800 000 случаев ВО, что составляет ежегодно 500–550 случаев на 100 тыс. населения. Более 90% заболевших составляют дети, примерно 70% – дети в возрасте от 1 года до 6 лет. По величине экономического ущерба от инфекционных болезней в Российской Федерации ВО находится на 2-м месте после гриппа и ОРВИ [7].
В большинстве случаев ВО протекает легко и заканчивается выздоровлением, однако у детей с ослабленным иммунитетом и взрослых она может иметь тяжелое клиническое течение, которое в ряде случаев приводит к необратимому повреждению центральной нервной системы [8]. Отмечено расширение клинического полиморфизма заболевания и нарастание доли крайне тяжелых и летальных форм. В научной литературе описаны случаи развития постветряночных васкулитов, в том числе гигантоклеточного артериита, энцефалитов и менингоэнцефалитов [8–13]. В ряде исследований показана связь развития инсультов после перенесенной инфекции у взрослых и детей [14]. Наиболее тяжелые формы осложнений описаны у пациентов с ВИЧ-инфекций и онкологических больных, доля которых в современном обществе нарастает [15].
Особой группой риска являются беременные и новорожденные. При заболевании женщины ВО в последнем триместре беременности риски инфицирования новорожденного, развития летальных исходов у матери и ребенка многократно возрастают [16–18].
ВО имеет очень высокий индекс контагиозности, поэтому вероятность заболевания здоровых лиц в течение жизни составляет более 95%. Для современного эпидемического процесса заболевания характерно вовлечение всех возрастных групп и формирование эпидемических очагов в организованных коллективах. Особую эпидемиологическую значимость имеют вспышки ВО, возникающие в медицинских организациях, в частности, в перинатальных центрах, детских организованных коллективах и воинских частях [19–22].
Вакцинопрофилактика ВО в настоящее время является наиболее перспективным способом управления этой инфекцией. В России зарегистрированы 2 моновалентные вакцины, продемонстрировавшие высокую клиническую и эпидемиологическую эффективность при хорошем профиле безопасности. Отмечено различие в подходах к организации иммунизации против ВО в разных странах. В одних странах применяется универсальная тактика вакцинация всех детей в 1 год и на 6-й год жизни, в других реализуется селективная и/или постэкспозиционная иммунопрофилактика. Различаются и подходы к выбору интервала между введениями двух доз вакцины (стандартный, удлиненный, ускоренный). Каждая из указанных тактик иммунизации против ВО имеет свои преимущества и ограничения [23–27].
Цель исследования – изучение современных эпидемиологических рисков распространения ВО и определение подходов к выбору тактики иммунизации.
Материалы и методы
Исследование носило ретроспективный описательный характер. Были проанализированы:
- данные форм федерального статистического наблюдения № 23 «Сведения о вспышках инфекционных заболеваний» за 2018–2020 гг., представленные управлениями Роспотребнадзора Уральского и Сибирского федеральных округов (УФО и СФО) (36 единиц информации);
- копии «Актов эпидемиологического расследования очагов инфекционных (паразитарных) болезней с установлением причинно-следственной связи», представленные этими же организациями (28 единиц информации);
- государственные доклады управлений Роспотребнадзора в субъектах РФ, входящих в УФО и СФО, за 2018–2020 гг. (36 единиц информации);
- государственные доклады «О состоянии санитарно-эпидемиологического благополучия населения Российской Федерации» за 2018–2020 гг.;
- данные годовых отчетов медицинских организаций Свердловской области за 2011–20218 гг.
Применяли эпидемиологический и статистический методы исследования.
Для обработки данных использовали табличный процессор Statistica, версия 10. Статистическую обработку полученных результатов проводили с применением общепринятых методов биостатистики с расчетом относительных величин, экстенсивных и интенсивных показателей заболеваемости. Достоверность различий оценивали по t-критерию Стьюдента. Различия считали достоверными при р < 0,05.
Результаты и обсуждение
Современные эпидемиологические риски распространения ВО определяются высоким уровнем заболеваемости, вовлечением в эпидемический процесс всех возрастных групп населения, формированием эпидемических очагов в детских и взрослых организованных коллективах, в том числе таких как медицинские организации, организации социального обслуживания населения, воинские коллективы.
Согласно ведущим эпидемиологическим теориям (Л.В. Громашевский, 1962; В.Д. Беляков, 1989), наиболее эффективным воздействием на эпидемический процесс ВО следует считать воздействие на 3-е звено – восприимчивый организм (человека). Иммунизация населения против VZV при этом является технологией выбора, так как дает значительный эффект в короткие сроки при оптимальных затратах. При этом следует учитывать соответствие выбранной тактики иммунизации существующей эпидемической ситуации по заболеваемости ВО и решаемым задачам.
С эпидемиологической точки зрения наиболее эффективной тактикой является массовая универсальная иммунизация против ВО всего детского населения. Наиболее эффективен такой подход при наличии высоких рисков эпидемического распространения VZV на территории муниципального образования (субъекта РФ). По данным Роспотребнадзора, объем иммунизации против ВО в большинстве регионов России остается незначительным, что, безусловно, влияет на уровень заболеваемости.
В субъектах УФО уровень заболеваемости ВО в 2018 г. был значительно выше, чем в целом по Российской Федерации (см. таблицу).
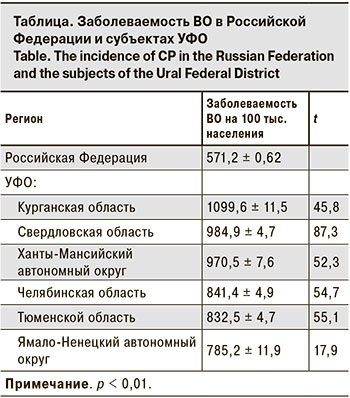
Наиболее интенсивным эпидемический процесс ВО был среди детского населения: уровень заболеваемости превышал аналогичный показатель среди взрослых в 50–80 раз.
Благодаря противоэпидемическим мероприятиям, проводимым в рамках борьбы с пандемией, вызванной новой коронавирусной инфекцией (COVID-19) во многих регионах снизился и уровень заболеваемости ВО. Так, по итогам 2020 г. в субъектах УФО произошло снижение заболеваемости ВО на 43% (в 2020 г. – 453,0 на 100 тыс. населения, в 2019 г. – 793,2), в субъектах СФО – на 29% (2020 г. – 381,1 на 100 тыс. населения, 2019 г. – 537,7). Однако актуальность этой инфекции сохранилась для всех субъектов РФ, даже в условиях масочного режима и снижения частоты и интенсивности социальных контактов.
В допандемический период эффективность универсальной вакцинации детского населения наглядно продемонстрирована в городском округе Качканар Свердловской области, где в 2011–2012 гг. была проведена вакцинация против ВО всего детского населения. Результатом стало снижение уровня заболеваемости ВО в 27 раз по сравнению с предыдущим периодом (в 2010 г. – 556,5 ± 36,5 на 100 тыс. населения, в 2012 г. – 20,6 ± 7,1; t = 14,4; p < 0,01) и в 35 раз по сравнению со среднемноголетним уровнем (СМУ – 722,1 ± 41,8 на 100 тыс. населения, 2012 г. – 20,6 ± 7,1; t = 16,5; p < 0,01).
Необходимым условием эффективности вакцинации является сохранение постоянства объемов и охвата прививками. В этом же муниципальном образовании прекращение плановой иммунизации против ВО привело к прогрессирующему росту заболеваемости до эпидемического уровня в последующие 3–4 года (2012 г. – 20,6 ± 7,1 на 100 тыс. населения, 2016 г. – 655,3 ± 40,5; t = 15,4; p < 0,01).
Снизить риск эпидемического распространения ВО на территории муниципального образования (субъекта РФ) возможно только при реализации универсальной плановой иммунизации против нее всего детского населения. В инструкции по применению вакцины рекомендовано двукратное введение препарата. Целесообразно использовать длинный интервал между введением доз вакцины против ВО, максимально комбинируя его с проведением предусмотренной Национальным календарем профилактических прививок вакцинации против кори/паротита/краснухи.
Однако реализация универсальной тактики иммунизации против ВО в рамках региональных программ не всегда возможна в силу экономических причин, в связи с этим необходимо проводить селективную и постэкспозиционную профилактику.
Широкое распространение в популяции инфекции, вызванной VZV, создает возможность заноса инфекции в социально значимые организованные коллективы детей и взрослых. Эпидемиологическая значимость организованных коллективов определяется наличием в них групп риска по развитию осложнений и тяжелому клиническому течению ВО. В первую очередь к ним относятся медицинские организации, учреждения стационарного социального обслуживания и воинские коллективы.
По данным годовых отчетов медицинских организаций Свердловской области, за 8-летний период наблюдения (2011–2018 гг.) было зарегистрировано 620 случаев заноса ВО в отделения больниц (в среднем 77 случаев в год; 95% ДИ 70–85). Чаще всего заносы происходили в отделения педиатрического профиля (301 случай или 48,6%), что объяснимо с точки зрения распространенности заболевания среди детей. На 2-м и 3-м месте были отделения терапии (163 случая или 26,3%) и хирургии (82 случая или 13,2%), оказывающие медицинскую помощь взрослому населению, на 4-м – отделения реанимации и интенсивной терапии (62 случая или 10,0%).
Периодически возникали эпидемиологически сложные ситуации в родильных домах, когда в процессе родов либо в раннем послеродовом периоде у женщин разворачивалась клиника ВО (12 случаев или 1,9%). Такие ситуации требовали проведения дополнительных противоэпидемических мероприятий, зачастую сопровождавшихся закрытием отделений на карантин и прекращением оказания населению плановой медицинской помощи (рис. 1, см. на вклейке).
Особенно сложная ситуация сложилась при заносе ВО в Областной перинатальный центр, когда встал вопрос о прекращении оказания специализированной неонатальной и акушерской помощи женщинам целого региона. В результате двух последовательных заносов контакт с источниками инфекции был отмечен у 387 чел., в том числе среди медицинского персонала – у 246 чел., среди пациенток отделения патологии беременных – у 66 чел., отделения патологии новорожденных – у 34 чел. и у 41 новорожденного.
Потебовалось проведение масштабных противоэпидемических мероприятий и возникла необходимость вакцинации работников учреждений родовспоможения и детства против ВО как варианта селективной иммунизации, что и было реализовано.
Учитывая это, влияние на риск заноса ВО в социально значимые коллективы должно осуществляться путем плановой иммунизации ряда эпидемиологически значимых контингентов (медицинских работников, работников детских и социальных учреждений и т. п.). При этом реализуется селективная тактика вакцинации, цель которой – защитить лиц из групп риска. Поскольку вакцина против ВО вводится двукратно, для профилактики ВО «прорыва» следует выбирать короткий интервал между вакцинациями: от 6 нед. до 6 мес. Необходимо учитывать, что такая тактика вакцинации не оказывает значимого влияния на уровень заболеваемости ВО в регионе и течение эпидемического процесса.
Однако VZV обладает высоким базовым репродуктивным числом (R0 = 10–12), что обеспечивает большую вероятность распространения инфекции при заносе в организованные коллективы. Наиболее часто эпидемические очаги ВО формировались в детских образовательных организациях. Так, в Курганской области в 2018 г. было зарегистрировано 130 очагов в детских дошкольных организациях, общее число пострадавших – 1907 чел. (14–15 чел. в очаге), в Челябинской области – 55 очагов и 424 пострадавших (7–8 чел. в очаге). В Свердловской области в детских дошкольных образовательных организациях были зарегистрированы вспышки ВО с вовлечением в эпидемический процесс до 90% списочного состава и продолжительностью существования очага более 100 дней.
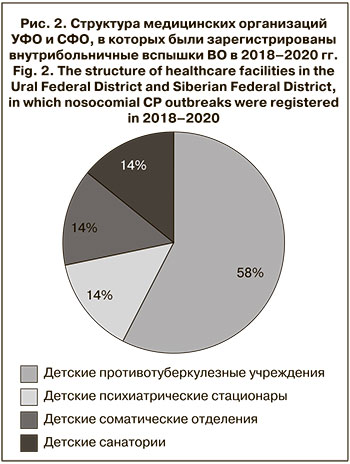
Медицинские организации также являются объектами риска по возникновению вспышек ВО. В медицинских организациях УФО и СФО в 2018–2020 гг. было зарегистрировано 7 случаев групповой и вспышечной заболеваемости, в которых пострадали в общем 82 чел.
Большая часть вспышек (57%) возникла в детских противотуберкулезных учреждениях, по 1 вспышке отмечено в детском психиатрическом стационаре, детском соматическом отделении Центральной районной больницы и детском санатории. Средняя продолжительность существования внутрибольничного очага составила 21 день, максимальная – 34 дня. Источником инфекции во всех случаях были пациенты с «пропущенным случаем» ВО, клиническая картина при этом была типична: везикулезные высыпания на коже – у 83,3% заболевших, повышение температуры тела > 37,5 °С – у 22,9%, катаральные явления – у 12,5%.
Анализ качества противоэпидемических мероприятий при ликвидации внутрибольничных очагов ВО позволяет сделать вывод о недооценке постэкспозиционной вакцинопрофилактики. В большинстве описанных случаев она не проводилась, что увеличило риски распространения инфекции.
В то же время опыт ликвидации очага ВО в Областном перинатальном центре Свердловской области говорит об успешности данной технологии при условии ее реализации в первые 96 ч от момента контакта с больным. При проведении первичных противоэпидемических мероприятий по случаю заноса ВО в перинатальный центр было выявлено большое число контактных лиц среди персонала (246 чел.), что ставило под сомнение возможность работы учреждения в плановом режиме.
Значительная часть контактных лиц считали себя переболевшими ВО (по данным анкетирования – 96,8% от числа персонала) и не соглашалась на проведение прививок по эпидемическим показаниям. После исследования напряженности иммунитета к VZV среди персонала было выявлено 7,1% серонегативных лиц. В отдельных возрастных группах доля серонегативных достигала 9–10% (20–29 лет – 8,8%, 40–49 лет – 10,4%, 50–59 лет – 9,1%), что, безусловно, создавало условия для реализации эпидемического потенциала VZV. Полный охват постэкспозиционной вакцинацией серонегативных к VZV лиц в течение 96 ч с момента контакта позволил предотвратить распространение инфекции в очаге и сохранить работоспособность персонала перинатального центра. Нежелательных явлений в поствакцинальном периоде отмечено не было.
Влиять на риск возникновения групповой и вспышечной заболеваемости ВО в организованных коллективах можно также путем постэкспозиционной иммунизации, цель которой – защитить лиц, контактных с больным ВО. Такая тактика должна быть реализована в первые 72–96 ч от момента контакта с больным. Принципы ее проведения и результаты схожи с таковыми при селективной профилактике.
Выводы
1. Современные эпидемиологические риски распространения ВО определяются широким распространением этой инфекции в популяции, способностью формировать крупные эпидемические очаги, в том числе в социально значимых организованных коллективах с вовлечением в эпидемический процесс разных возрастных и социальных групп населения.
2. С целью снижения рисков распространения ВО могут быть реализованы различные тактики иммунизации восприимчивого населения. Так, в ожидании включения вакцинации против ВО в Национальный календарь профилактических прививок Российской Федерации необходимо активно внедрять региональные программы вакцинации, которые могут быть нацелены на селективную иммунизацию групп риска инфицирования и развития тяжелых клинических форм и осложнений, и постэкспозиционную профилактику в организованных коллективах для предупреждения вспышечной заболеваемости. При этом следует учитывать, что данный подход не окажет значимого влияния на развитие эпидемического процесса как в отдельном регионе, так и в стране в целом.
3. Универсальная тактика иммунизации против ВО всего детского населения с максимальным совмещением введения вакцины против ВО с вакцинами Национального календаря профилактических прививок (корь/паротит/краснуха) является тактикой выбора для реализации контроля заболеваемости на уровне города, региона, страны. При этом необходимо проводить постоянную работу по поддержанию высокого охвата подлежащих лиц прививками против ВО.


