Туберкулез (ТБ) с множественной лекарственной устойчивостью (МЛУ), как и ко-инфекция ВИЧ/ТБ, на настоящий момент представляют собой основные препятствия на пути искоренения ТБ согласно стратегии ВОЗ [1]. По мнению многих исследователей, МЛУ-ТБ среди лиц, живущих с ВИЧ, встречается чаще, чем среди ВИЧ-негативных [2, 3]. Существует немало теорий о причине такой эпидемиологической картины. Наиболее значимой нам представляется циркуляция лекарственно устойчивых штаммов среди лиц, наиболее подверженных заражению ВИЧ, и развитие активного ТБ вскоре после первичного инфицирования ввиду иммунодефицита.
Наличие МЛУ у штаммов микобактерий существенно ухудшает исходы лечения ТБ [4]. Согласно результатам последнего проведенного метаанализа, наличие у пациентов ВИЧ-инфекции еще более компрометирует результаты терапии [5]. К возможным причинам неблагоприятных исходов среди лиц, живущих с ВИЧ, относятся позднее начало терапии в связи с трудностями диагностики при более прогрессивном течении заболевания; вынужденная полипрагмазия и, как результат, более частые отмены препаратов из-за нежелательных явлений (НЯ); наличие и тяжесть других оппортунистических инфекций; более частое сочетание ТБ с другими хроническими заболеваниями (например, вирусными гепатитами), а также с алкогольной и наркотической зависимостью; более низкая приверженность терапии ввиду нерешенных социально-экономических проблем.
Согласно всем современным руководствам по лечению ВИЧ-инфекции и ТБ [6–8], большинство препаратов, применяемых для терапии МЛУ-ТБ, не имеют значимых лекарственных взаимодействий с АРТ и, следовательно, могут применяться у данной группы пациентов в своих обычных дозировках. Исключения представляют бедаквилин и деламанид. По имеющимся на сегодняшний день данным (а это исследования, включавшие небольшое число пациентов), бедаквилин не рекомендуется назначать ни с ингибиторами протеазы (ИП) [9], ни с ненуклеозидными ингибиторами обратной транскриптазы (ННИОТ) [10]. В отношении деламанида значимые изменения фармакокинетики отмечались лишь при сочетании его с ИП [11]. В то же время достаточно хорошо описана перекрестная токсичность противотуберкулезной терапии (ПТТ) и АРТ. Ранее нами было показано, что исследования, посвященные отдельным группам НЯ при лечении МЛУ-ТБ, обычно выявляли их большую частоту среди ВИЧ-позитивных пациентов, в то время как в работах, посвященных всему спектру НЯ, данные были противоречивы [12].
Целью данного исследования стало изучение распространенности и спектра НЯ, возникающих на фоне сочетанной терапии МЛУ-ТБ и ВИЧ-инфекции, и их взаимосвязь с применяемыми схемами АРТ.
Материалы и методы
На основании изучения историй болезни было проведено ретроспективное когортное исследование пациентов старше 18 лет с ВИЧ-инфекцией, зарегистрированных на лечение по 4-му режиму химиотерапии ТБ в период 2014–2016 гг. в г. Владимире и Владимирской области.
Критериями включения были: наличие ВИЧ-инфекции (положительный результат иммуноблотинга), терапия ТБ с доказанной или предполагаемой лекарственной устойчивостью и прием антиретровирусных препаратов. В исследование не были включены пациенты с ко-инфекцией МЛУ-ТБ и ВИЧ, если они не получали АРТ в период наблюдения.
Для пациентов, получавших стационарное лечение в 2014–2017 гг., проанализирован период госпитального лечения. В небольшом числе случаев, когда пациента не госпитализировали для начала ПТТ, в анализ был включен весь период ПТТ. Критерии наличия и степени тяжести НЯ оценивали в соответствии с клиническими рекомендациями по лечению ТБ у больных ВИЧ-инфекцией [8].
Исследование было одобрено Комитетом по этике Медицинского института РУДН. Поскольку исследование проводилось ретроспективно, согласия пациентов не требовалось. Вся информация из карт стационарного и амбулаторного наблюдения в обезличенном виде (с присвоением идентификационного номера каждому пациенту) вносилась в специально разработанную базу данных.
Статистический анализ проводили, используя программу IBM SPSS Statistics 25.0 (IBM SPSS Statistics for Windows, SPSS Inc., Chicago, IL, USA). Для оценки достоверности различий между группами использовали критерии Фишера, χ2 в соответствии с рекомендациями, а также U-критерий Манна–Уитни для сравнения уровня CD4-лимфоцитов в группах. Различия считали статистически достоверными при p < 0,05.
Результаты
В анализ были включены 60 пациентов, из них 14 (23,3%) женщин. На момент начала лечения ТБ пациенты были в возрасте от 27 до 52 лет, средний возраст составлял 36,4 ± 11,2 года.
У 43 (64,2%) пациентов ТБ был выявлен впервые, остальные представляли группу повторно леченных. Формы ТБ распределились следующим образом: инфильтративный – у 24 (40%) пациентов, диссеминированный – у 19 (31,7%), очаговый ТБ легких – у 7 (11,7%), казеозная пневмония – у 4 (6,7%), туберкулезный плеврит без поражения легочной ткани – у 2 (3,3%). По 1 (1,65%) случаю приходилось на цирротический ТБ, туберкулому, ТБ внутригрудных лимфатических узлов и костно-суставной ТБ.
Среди пациентов с первичным поражением легких у 13 (21,7%) заболевание осложнялось внелегочным поражением, у 8 (13,7%) – туберкулезным плевритом. Среди внелегочных локализаций наиболее часто встречались ТБ периферических лимфоузлов, костно-суставной ТБ – по 4 (6,7%) случая, туберкулез кишечника – 3 (5%), по 1 (1,7%) случаю приходилось на ТБ мягких тканей, туберкулезный орхоэпидидимит и туберкулезный менингоэнцефалит. 1 случай внелегочного ТБ (ТБ спондилит) не сопровождался легочным поражением. Период наблюдения составил в среднем 86,1 дня (медиана – 67,5 дня [29–110]).
Большинство пациентов имели выраженную иммуносупрессию, среднее количество СD4-лимфоцитов – 201 клетка/мкл (медиана – 186 клеток/мкл [51,5–308]). 25 (41,7%) человек получали АРТ более 30 дней до установления МЛУ-ТБ, 11 (18,3%) АРТ была назначена менее чем за 1 мес. до начала ПТТ, 8 (13,3%) начали АРТ в течение 2 нед. после адекватной ПТТ, при этом у 3 из них количество CD4-лимфоцитов составляло более 50 клеток/мкл. Остальным 16 (26,7%) пациентам АРТ была назначена позднее. Немаловажно, что среди 25 пациентов, получавших АРТ более 1 мес. на момент начала ПТТ, только у 7 (28%) была неопределяемая вирусная нагрузка (у 1 пациента сведения отсутствовали). Возможно, это связано с низкой приверженностью терапии: у 18 пациентов (30%) в исследованной когорте в анамнезе отмечены перерывы в приеме антиретровирусных препаратов.
ПТТ и АВТ назначали в соответствии с действовавшими на тот период клиническими рекомендациями [13, 14]. В табл. 1 представленыы препараты, входившие в схему ПТТ, в табл. 2 – препараты АРТ. Пациенты, вошедшие в исследуемую группу, не получали бедаквилин. 39 (65%) пациентов получали АРТ на основе ННИОТ (1-я группа), 21 (35%) – на основе ИП (2-я группа). При этом наиболее часто применявшимся препаратом в 1-й группе был эфавиренз (п = 36; 92,3%), во 2-й – лопинавир/ритонавир (п = 14; 66,7%).
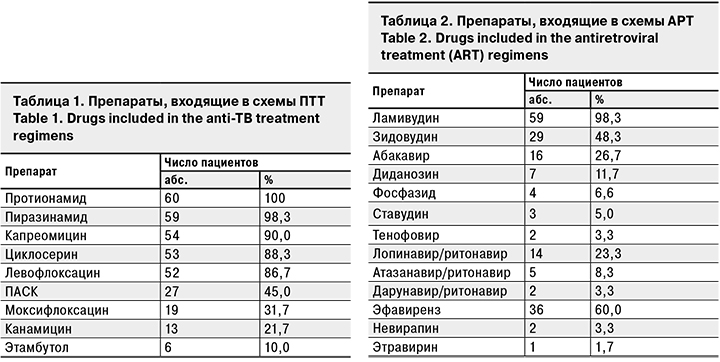
За период лечения наличие любых НЯ мы выявили у 38 (63,3%) пациентов, НЯ 3–4-й степени тяжести – у 11 (18, 3%). Частота любых НЯ в среднем на 1 пациента составила 1,2 случая, НЯ 3–4-й степени тяжести – 0,28.
Среди НЯ 3–4-й степени тяжести отмечали значительное повышение мочевой кислоты у 2 пациентов (только в 1 случае оно сопровождалось артралгиями), изолированное повышение печеночных трансаминаз – у 2, по 1 случаю приходилось на отек Квинке, панкреатит, эпиприступ, геморрагический синдром, анемию. У 2 пациентов развилась клиническая картина декомпенсации цирроза печени (в 1 случае с преобладанием холестатического синдрома, еще в 1 – гепаторенального). Оба пациента до начала ПТТ страдали хроническим гепатитом (С и В соответственно) с признаками цирроза печени. За исключением последних 2 случаев НЯ были успешно купированы на фоне патогенетической терапии или отмены/замены препаратов. Один пациент с циррозом печени скончался в туберкулезном стационаре, второй – после перевода в инфекционный стационар. Связь с приемом ПТТ (пиразинамида) наиболее явно прослеживалась в случае с выраженной аллергической реакцией. Остальные НЯ, за исключением геморрагического синдрома, анемии и панкреатита, наиболее вероятно были связаны с приемом противотуберкулезных препаратов. Анемия, скорее всего, была вызвана приемом фосфазида.
У 7 (11,7%) пациентов потребовалась полная отмена как минимум 1 препарата в схеме ПТТ в связи с НЯ, у 5 (8,3%) – временная отмена всей схемы. Отмену АРТ в нашем исследовании регистрировали гораздо реже: у 2 (3,3%) пациентов были временно отменены все противовирусные препараты, после чего в 1-м случае произведена полная смена схемы терапии, во 2-м возобновлена та же схема. Чаще проводили коррекцию схемы АРТ: у 6 (10%) пациентов в связи с цитопенией на зидовудин и фосфазид они были заменены на другие препараты группы НИОТ, у 2 (3,3%) эфавиренз был заменен на невирапин ввиду НЯ со стороны центральной нервной системы.
Структура наблюдаемых НЯ представлена на рис. 1.
Среди описанных НЯ лишь миелотоксические проявления можно в первую очередь связать с приемом АРТ. Гастроинтестинальные, гепатотоксические, аллергические и психоневрологические НЯ (куда входили НЯ со стороны как центральной, так и периферической нервной системы) могут быть вызваны приемом как ПТТ, так и АРТ. Стоит отметить, что в данной когорте не было возможностей для регулярного проведения аудиометрии и лабораторного контроля гормонов щитовидной железы, в связи с чем возможно занижение частоты НЯ.
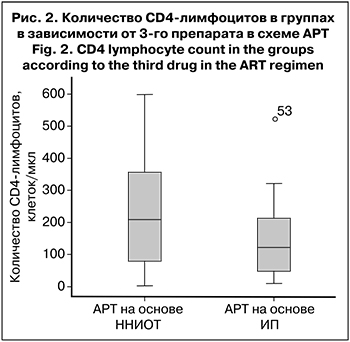 Мы разделили пациентов на 2 группы в зависимости от 3-го препарата в схеме АРТ (ННИОТ или ИП). Было выявлено, что любые НЯ, в том числе 3–4-й степени тяжести, достоверно чаще встречались в группе пациентов, получавших АРТ на основе ИП – 80,9 против 53,8% (р = 0,038) и 52,4 против 15,3% (р = 0,002) соответственно. Возможно, это было связано с более выраженной иммуносупрессией во 2-й группе пациентов, так как согласно рекомендациям по лечению ВИЧ-инфекции, ИП чаще назначают пациентам с выраженным снижением количества CD4-лимфоцитов. Действительно, во 2-й группе медиана количества CD4-лимфоцитов. составила 122 клетки/мкл (45–223), в 1-й – 208 клеток/мкл (63–72) (рис. 2). Однако это различие оказалось статистически недостоверным (р = 0,106).
Мы разделили пациентов на 2 группы в зависимости от 3-го препарата в схеме АРТ (ННИОТ или ИП). Было выявлено, что любые НЯ, в том числе 3–4-й степени тяжести, достоверно чаще встречались в группе пациентов, получавших АРТ на основе ИП – 80,9 против 53,8% (р = 0,038) и 52,4 против 15,3% (р = 0,002) соответственно. Возможно, это было связано с более выраженной иммуносупрессией во 2-й группе пациентов, так как согласно рекомендациям по лечению ВИЧ-инфекции, ИП чаще назначают пациентам с выраженным снижением количества CD4-лимфоцитов. Действительно, во 2-й группе медиана количества CD4-лимфоцитов. составила 122 клетки/мкл (45–223), в 1-й – 208 клеток/мкл (63–72) (рис. 2). Однако это различие оказалось статистически недостоверным (р = 0,106).
В отношении наиболее часто встречавшихся НЯ (гастроинтестинальных и гепатотоксических) не были доказаны статистически достоверные различия между группами (р =0,532 и р=0,226 соответственно). Однако следует отметить, что, в отличие от поражения печени по типу цитолиза (с преимущественным повышением уровня трансаминаз), холестатический тип (с преимущественным повышением уровня билирубина) встречался исключительно во 2-й группе (3 случая). Также не удалось обнаружить статистически достоверных различий в частоте других, более редких НЯ, что связано с относительно небольшим числом зарегистрированных случаев. Лишь при сравнении частоты анемии между группами значение p было на границе значимости (p = 0,061), однако данное НЯ более вероятно было связано с различиями в нуклеозидной основе АРТ.
Обсуждение
В ходе исследования при анализе данных возникли некоторые сложности. Первая – это отсутствие универсального подхода к определению и регистрации случаев НЯ исследователями, что приводит к трудностям при сопоставлении данных. Часто трудно разделить НЯ, не связанные преимущественно с ПТТ или АРТ. В нашем исследовании большинство НЯ были в первую очередь вызваны ПТТ, так как согласно данным литературы и рекомендациям по применению лекарственных препаратов, описываемые группы НЯ чаще встречаются на фоне ПТТ. Косвенно это подтверждается тем, что при временной или постоянной отмене противотуберкулезных препаратов почти всегда удавалось купировать НЯ. Значимой группой НЯ, которую мы отнесли к вызванным АРТ, были миелотоксические, что связано в основном с использованием зидовудина и фосфазида. На настоящий момент эти препараты уже не относятся к первой линии АРТ, в нашей стране их замена стала более доступной с появлением дженериков тенофовира и, соответственно, более широким включением этого препарата в схемы АРТ.
63,3% пациентов на стационарном этапе лечения отмечали те или иные НЯ, в среднем на 1 пациента зарегистрировано более 1 случая НЯ, что оказалось выше, чем в большинстве зарубежных исследований. Так, по данным мета-анализа, проведенного учеными из США [15], общая частота НЯ при одновременном проведении АРТ и терапии МЛУ-ТБ составила 31%.
Структура НЯ, по нашим данным, в целом была сопоставима с результатами других исследований [16–18]. Так, наиболее часто встречавшимися НЯ во всех перечисленных работах были гастроинтестинальные (около 45%). По сравнению с данными S.S. Shin и соавт. [19] , в нашей когорте реже встречались НЯ, связанные с центральной и периферической нервной системой. В их исследовании периферическая нейропатия отмечена у 37% пациентов, психиатрические нарушения – у 28%, в то время как по нашим данным психоневрологические нарушения составили лишь 5,5% от всех зарегистрированных НЯ. В то же время результаты, полученные K. Schnippel и соавт. [18], сопоставимы с нашими, где выявлено 6,7% психоневрологических нарушений. Мы реже регистрировали гипотиреоидизм и снижение слуха, что было вызвано ограниченными возможностями мониторинга. Гепатотоксические явления в исследовании, проведенном в Аргентине [17], как и в нашей когорте, были нередки – до 14% от всех НЯ среди ВИЧ-положительных пациентов, однако в индийской когорте не было отмечено ни одного случая гепатотоксичности [16]. Данный факт может быть связан как с особенностями популяции пациентов (частота хронических гепатитов, употребления алкоголя и наркотиков), таки с возможностями лабораторного мониторинга. Учитывая, что многие исследования, посвященные проблеме сочетания АРТ и ПТТ, проводятся в странах со средним и низким уровнем дохода, где не всегда возможно соблюдение полного рекомендованного лабораторного и инструментального мониторинга [20], это не позволяет однозначно сравнивать результаты исследований.
При этом в нашем исследовании серьезные НЯ, приведшие к смерти пациентов, были связаны с декомпенсацией имевшегося цирроза в исходе хронических гепатитов, что подчеркивает актуальность проблемы в российской когорте пациентов.
По нашим данным, полная отмена одного или нескольких препаратов чаще требовалась в отношении ПТТ, чем АРТ (20% против 3,3%). Учитывая более частую связь НЯ с ПТТ и более быстрое развитие лекарственной устойчивости ВИЧ по сравнению с M. tuberculosis, клинические рекомендации предлагают при наличии выраженного НЯ, не купируемого симптоматической терапией, когда неизвестен вызвавший его препарат, в первую очередь проводить временную отмену ПТТ [8]. По данным исследования, проведенного в Томске среди пациентов с моноинфекцией МЛУ-ТБ отмена препаратов в схеме ПТТ требовалась существенно чаще и составила 28,7% [19] против 11,7% в нашем исследовании. Но в работе томских коллег проанализирован весь период лечения, в то время как нам удалось исследовать лишь НЯ, возникшие преимущественно на стационарном этапе лечения.
Следует отметить низкую приверженность АРТ в исследуемой группе пациентов: 30% имели в анамнезе упоминания о перерывах в приеме препаратов. У 18,3% пациентов АРТ назначалась/возобновлялась в течение 1 мес. до установления диагноза ТБ. По-видимому, пациенты, чувствуя ухудшение, обращались за медицинской помощью, и им назначали АРТ, хотя в данной ситуации при наличии жалоб целесообразнее было провести дообследование.
С чем былая связана более высокая частота НЯ в группе пациентов, получавших ИП, окончательно неясно, этот факт требует дальнейшего изучения.
Заключение
Проведенное исследование еще раз продемонстрировало частое развитие НЯ среди пациентов, получающих лечение МЛУ-ТБ и АРТ. В то же время доля пациентов, которым потребовалась отмена препаратов в связи с НЯ, была ниже, чем по данным других авторов.
Учитывая распространенность НЯ в этой группе, важными являются своевременная их регистрация и грамотная коррекция, а в идеале – их профилактика, что может улучшить исходы лечения. Представляется перспективной разработка универсальных алгоритмов выявления и регистрации НЯ у пациентов, получающих комбинированную терапию МЛУ-ТБ и ВИЧ-инфекции, проведение дальнейших исследований в этой области для уточнения влияния НЯ на исходы терапии и выроботку рекомендаций по профилактике и мониторингу НЯ. Способствовать улучшению исходов в данной группе пациентов могут также внедрение современных схем как АРТ, так и ПТТ: отказ от зидовудина и фосфазида у пациентов с ко-инфекцией, широкое применение новых и перепрофилированных противотуберкулезных препаратов, а также повышение доступности терапии хронических гепатитов.


