Малярия – одна из наиболее широко распространенных и опасных инфекций [1, 2]. По данным ВОЗ, малярией ежегодно болеют около 660 000 человек, около 330 000 погибают [3, 4]. Более 99% случаев смерти от малярии приходится на долю тропической формы заболевания, вызываемой Plasmodium falciparum (P. falciparum). Эта инфекция представляет серьезную проблему для жителей развитых стран, по служебной или личной необходимости выезжающих в эндемичные регионы [5]. В РФ ежегодно регистрируются случаи гибели лиц, заразившихся малярией, вызванной P. falciparum, при посещении стран Африки, Юго-Восточной Азии, Южной Америки, островов Океании [3, 5]. Причинами этого служит высокая вирулентность P. falciparum, а также интенсивное формирование у паразитов устойчивости к действию противомалярийных препаратов [3, 6]. Если осложненное течение малярии, вызванной высоковирулентными возбудителями, может быть предупреждено своевременной диагностикой и ранним началом этиотропной терапии, то лекарственная резистентность плазмодиев до настоящего времени остается нерешенной проблемой для практической медицины [3, 5, 7]. Ситуация осложняется ограниченным перечнем противомалярийных препаратов, прошедших государственную регистрацию в РФ. Нередко больным малярией, вызванной P. falciparum, вынужденно назначают лишь 1 этиотропный препарат, недостаточно эффективный в отношении лекарственноустойчивых паразитов, в то время как отход от практики монотерапии низкоэффективными средствами стал общемировой нормой лечения малярии [6–9].
Цель работы – изучение и обобщение данных об особенностях метаболизма P. falciparum, фармакологическом действии противомалярийных препаратов и молекулярно-генетических механизмах формирования лекарственной резистентности возбудителей малярии, вызванной P. falciparum, и определение современных тенденций научных исследований в этой области.
Такое положение во многом объясняется недостатком у врачей и организаторов системы здравоохранения знаний в области фармакологии малярии и лекарственной резистентности возбудителей. В этой ситуации становится актуальным формирование у медицинских специалистов современных представлений о механизмах действия лекарственных препаратов, а также молекулярно-генетических механизмах, лежащих в основе формирования лекарственной резистентности малярийных плазмодиев.
Были использованы материалы базы данных PlasmoDB (Plasmodium Data Base), содержащей сведения о геноме малярийных плазмодиев.
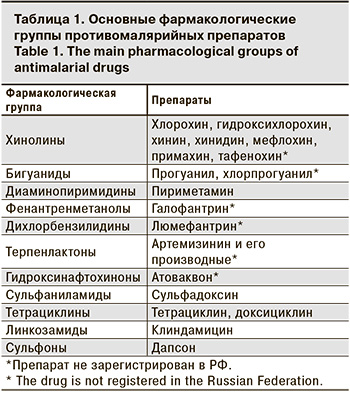
Арсенал этиотропных средств для лечения малярийной инфекции насчитывает более 20 препаратов, которые относятся к разным фармакологическим группам, отличающимся избирательностью воздействия на основные метаболические пути паразитарной клетки (табл. 1).
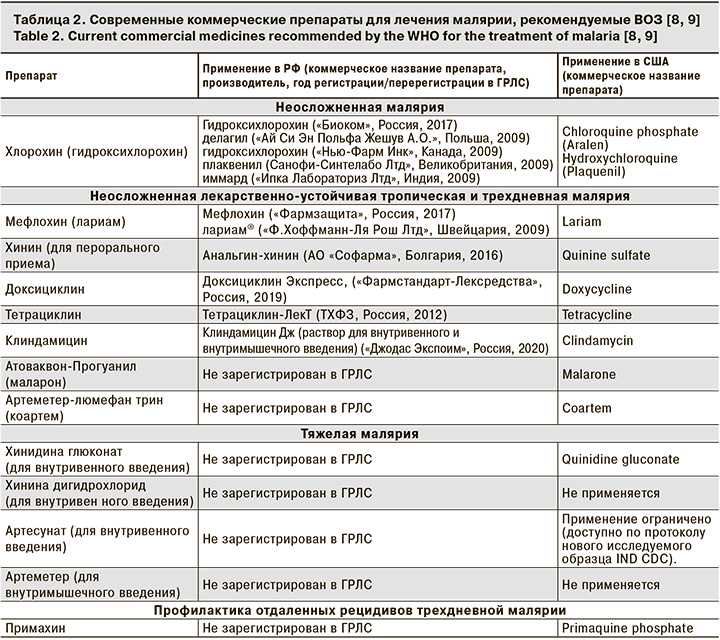
В настоящее время перечень препаратов, рекомендованных ВОЗ, ограничен и включает высокоэффективные, а также хорошо переносимые лекарственные средства с различным механизмом лекарственного воздействия на возбудителей (табл. 2).
Противомалярийные препараты могут блокировать или конкурентно замещать транспортные белки, а также ключевые паразитарные ферменты, катализирующие основные метаболические пути пластического и энергетического обмена [2, 10] (табл. 3).
Действие противомалярийных препаратов в основном направлено на эритроцитарные стадии развития паразитов. Интенсивное циклическое размножение плазмодиев предполагает высокую активность метаболических процессов, разобщение которых приводит к гибели паразитов [2]. Интенсивные обменные процессы обеспечиваются за счет поглощения паразитом гемоглобина из цитоплазмы пораженного эритроцита [8]. В пищеваритальной вакуоли плазмодия кровяной пигмент подвергается деградации. Питательным субстратом служит его белковая фракция. Железосодержащие компоненты пигмента (гематин, порфирин) токсичны для паразитов и метаболизируются их ферментными системами в нетоксичный гемозин, который откладывается в виде зернистости Маурэра и Шюфнера [11]. Разобщение этого метаболического пути с помощью химиопрепаратов приводит к накоплению токсичных продуктов и гибели плазмодиев. Трофозоит развивается внутри эритроцита, защищенный от цитоплазмы клетки хозяина мембраной паразитофорной вакуоли. В ее формировании участвует апикопласт паразита (видоизмененный аналог пластид), в строме которого происходит метаболизм жирных кислот II типа, необходимых для формирования структур биологической мембраны [12, 13]. Препараты, проникающие в апикопласт, нарушают эти процессы, что приводит к повреждению мембраны паразитофорной вакуоли, вызывая замедленный лечебный эффект [14, 15]. В процессе развития паразитов интенсивно реплицируется их ДНК. При этом существенно активируется фолатный путь биосинтеза нуклеиновых кислот [16]. Конкурентное замещение ключевых ферментов этого метаболического пути нарушает синтез нуклеиновых кислот и приводит к гибели всей генерации паразитов [8]. Интенсивное развитие сопровождается высоким уровнем биосинтеза полипептидов и фолдингом белков в эндоплазматическом ретикулюме паразитарной клетки. При этом уязвимым для действия противомалярийных препаратов становится мевалонатный путь биосинтеза изопреноидов, участвующих в процессах трансформации энергии [17]. Повышенный энергетический обмен поддерживается интенсивным клеточным дыханием и активным окислительным фосфорилированием в митохондрии паразита. В связи с этим блокирование химиопрепаратами цитохромоксидазного комплекса приводит к разобщению этих процессов и быстрому лечебному эффекту [18]. В процессе жизнедеятельности паразиты экспортируют вирулентные белки в клеточные структуры инфицированного эритроцита [19]. Препараты, нарушающие транспорт паразитарных белков, делают инфицированный эритроцит доступным для факторов клеточного и гуморального иммунитета, тем самым способствуя элиминации паразитов из крови.
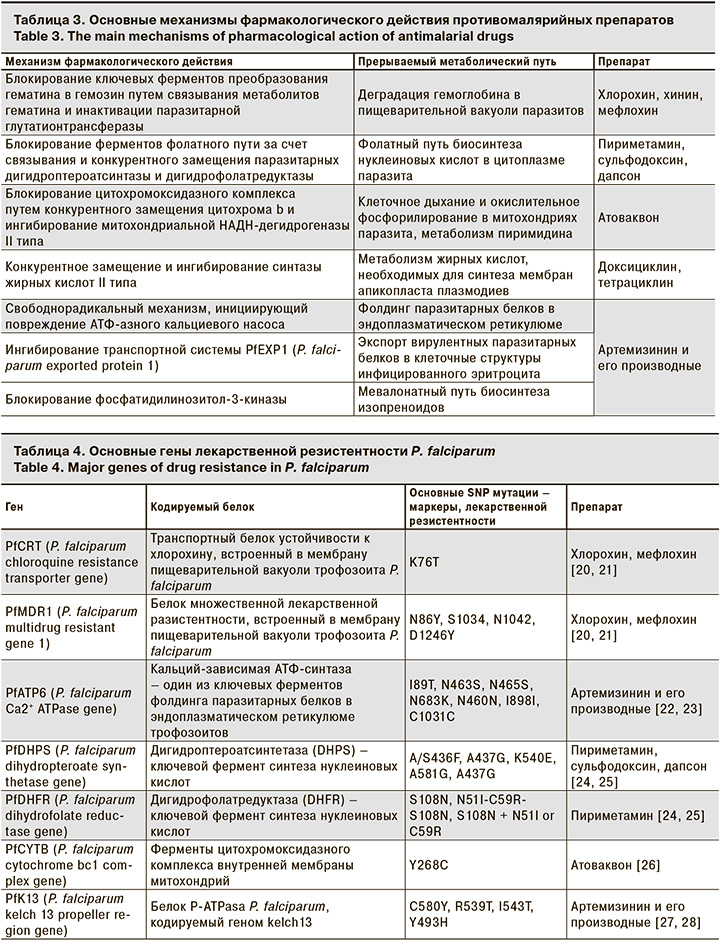
Несмотря на уязвимость метаболических процессов паразитарной клетки и разнообразие мишеней для действия противомалярийных препаратов, в настоящее время не существует ни одного абсолютно эффективного лекарственного средства [2, 8]. Формирование лекарственной устойчивости паразитов связано с их высокой пролиферативной активностью, а также наличием в геноме плазмодиев вариабельных областей, подверженных SNP-мутациям (single nucleotide polymorphisms) (табл. 4).
Механизм формирования лекарственной устойчивости паразитов зависит от конкретного механизма фармакологического действия противомалярийного препарата. Плазмодии приобретают резистентность в результате мутаций, изменяющих структуру белков и ферментов, которые служат мишенями химиопрепаратов [18, 19, 29].
Так, хлорохин, проникнув в пищеварительную вакуоль плазмодиев, взаимодействует с продуктами деградации гемоглобина, препятствуя преобразованию токсичного для паразитов порфирина в кристаллы гемозина [14, 30]. Формирование устойчивости плазмодиев к действию хлорохина связано с мутациями в генах PfCRT (P. falciparum chloroquine resistance transporter) и PfMDR1 (P. falciparum multi-drug resistant 1), кодирующих структуру транспортных белков мембраны пищеварительной вакуоли [20, 21]. Видоизмененные белки приобретает способность выводить хлорохин, снижая его концентрацию и препятствуя образованию токсичных комплексов.
Механизм противомалярийного действия других производных 4-аминохинолина (мефлохин, хинин, хинидин) сходен с действием хлорохина. Препараты образуют токсичные для паразита комплексы, связываясь с гемом. Устойчивость к их действию также зависит от особенностей транспортных белков PfCRT и PfMDR1, выводящих препараты и их производные через мембрану пищеварительной вакуоли паразитов [31–33]. При этом распространение паразитов, устойчивых к хинину, отличается более медленными темпами. Это указывает на то, что чувствительность P. falciparum к хинину определяется не только локусами PfMDR1 и PfCRT, но зависит и от особенностей других паразитарных генов [34, 35].
Сульфадоксин – сульфаниламидный препарат, применяемый в комбинации с пиреметамином (комбинированный препарат фансидар), ингибирует активность фермента дигидроптероатсинтазы (DHPS), который в цитоплазме паразитов участвует в биосинтезе фолиевой кислоты (витамин В9) [36]. Активная форма витамина В9, тетрагидрофолат (ТГФ), используется микроорганизмами для получения пуриновых азотистых оснований и синтеза собственных нуклеиновых кислот [25]. Точечные мутации в гене, кодирующем структуру DHPS, снижают сродство этого фермента к сульфадоксину [24].
Пириметамин, как и сульфодоксин, действует на бесполые эритроцитарные формы малярийных плазмодиев, подавляя биосинтез нуклеиновых кислот [25]. Пириметамин ингибирует активность дигидрофолатредуктазы (DHFR) и производство тетрагидрофолата [37]. Точечные мутации в гене, кодирующем структуру DHFR, снижают сродство фермента к пириметамину [24]. Паразиты, обладающие резистентностью к сульфодоксину и пиреметамину, не имеют широкого распространения. Однако в настоящее время эти препараты в качестве притивомалярийных средств применяются ограниченно в связи с их побочными действиями.
Атоваквон способен специфически связываться с цитохромом b плазмодиев и ингибировать его действие [38]. Потеря активности цитохромоксидазного комплекса приводит к коллапсу трансмембранного электрохимического потенциала и потере функции паразитарной митохондрии [39]. Помимо разобщения окислительного фосфорилирования атоваквон блокирует воспроизведение плазмодиями собственных нуклеиновых кислот, инактивируя PfDHPS, один из ключевых в биосинтезе пиримидина [40]. Развитие у P. falciparum устойчивости к атоваквону связано с возникновением точечных мутаций и замещением серина тирозином в 268 кодоне гена цитохрома, нуклеотидные последовательности которого входят в состав митохондриальной ДНК [26, 41].
Прогуанил – производное бигуанида. Действие его активного метаболита циклокуанила основано на ингибировании DHFR и блокировании фолатного пути биосинтеза азотистых оснований [42]. Прогуанил также связывает активные формы кислорода в митохондриальном матриксе, потенцируя тем самым действие атоваквона (в комбинированном препарате малорон) [40]. Точечные мутации в структуре DHFR способствуют формированию устойчивости паразитов к циклогуанилу [36, 43].
Доксициклин – антибактериальный препарат тетрациклиновного ряда. Предполагается, что он подавляет синтез паразитами белка на этапе элонгации, предотвращая связывание аминоацил-тРНК с малой рибосомальной субъединицей [8]. Доксициклин нарушает функцию апикопласта P. falciparum, что приводит к медленному, но выраженному антималярийному эффекту [12, 13, 15]. До настоящего времени нет сведений о формировании устойчивости малярийных паразитов человека к доксициклину. Ограниченное использование этого препарата для лечения малярии объясняется медленным формированием лечебного эффекта.
Производные артемизинина (артесунат, артеметер и др.) реализуют свободнорадикальный механизм блокирования метаболизма P. falciparum. Препарат взаимодействует с продуктами деградации гемоглобина, включающими оксид железа (II). Разрыв входящего в структуру действующего вещества эндопероксидного кольца приводит к образованию свободных радикалов, которые в свою очередь повреждают чувствительные белки, что приводит к гибели паразита [8]. Наиболее чувствителен к свободнорадикальному повреждению АТФ-азный кальциевый насос P-типа (P-ATPasa), что приводит к нарушению фолдинга паразитарных белков [22, 23]. В настоящее время установлено ингибирующее действие активной формы дигидроартемизинина на мембранную глутатион-S-трансферазу (glutathione-S-transferase – GST), которая обеспечивает естественный механизм защиты паразитарной клетки от цитотоксического эффекта продуктов деградации гемоглобина, поглощаемого плазмодиями [28]. Эффективным механизмом лекарственного действия производных артемизинина считается также ингибирование транспортной системы (Plasmodium falciparum exported protein 1 – PfEXP1), обеспечивающей экспорт вирулентных паразитарных белков в клеточные структуры инфицированных эритроцитов [44, 45].
Резистентность P. falciparum к действию производных артемизинина ассоциируется главным образом с множественными однонуклеотидными полиморфизмами (SNP) гена kelch-13, который кодирует полипептидную последовательность белка P-ATPasa P. falciparum. Точечные мутации, соответствующие пропеллерной области паразитарного белка, делают невозможным его специфическое связывание активными производными артемизинина [27].
ELQ-300 (Medicines for Malaria Venture – MMV) – перспективный инъекционный препарат длительного действия, первый в новом классе противомалярийных средств, известных как 4-хинолон -3-диариловые эфиры. ELQ-300 избирательно ингибирует митохондриальный электронный транспорт паразита и связанный с ним биосинтез пиримидина [46], тем самым предотвращая синтез нуклеиновых кислот. При этом ELQ-300 действует на консервативный участок цитохрома bc1, что позволяет предположить низкую вероятность формирования резистентности у паразитов. В доклинических исследованиях была показана высокая активность нового средства в отношении всех этапов жизненного цикла P. falciparum, P. vivax и P. knowlesi, в том числе с множественной лекарственной устойчивостью лабораторных штаммов и клинических изолятов. Вероятно, в случае успешного завершения клинических испытаний ELQ-300 может стать новым препаратом выбора для лечения и профилактики малярийной инфекции [47–49].
Таким образом, отмечается дальнейшее распространение малярийных плазмодиев, устойчивых к традиционным противомалярийным препаратам, а также формирование паразитарных штаммов, обладающих резистентностью к новым современным антипаразитарным средствам. Не существует противомалярийных препаратов, в отношении которых плазмодии не могут вырабатывать лекарственной устойчивости.
Основные механизмы лекарственной устойчивости связаны с генетической гетерогенностью возбудителей инфекции. В формировании устойчивости важное значение имеют SNP мутации по локусам генов PfCRT, PfMDR1, PfATP6, PfDHPS, PfDHFR, PfCYTB, а также рекомбинация генетического материала плазмодиев в ходе эритроцитарной шизогонии. Формированию резистентных штаммов и отбору мутаций лекарственной устойчивости способствует длительное персистирование паразитов в крови на фоне приема противомалярийных препаратов. Основными принципами этиотропного лечения малярии должны служить раннее начало лечения, проведение комбинированной терапии с использованием наиболее эффективных противомалярийных препаратов разных фармакологических групп в дозировках, обеспечивающих быструю элиминацию возбудителей из организма больного.
Дальнейшее изучение генетического полиморфизма малярийных плазмодиев по признаку лекарственной устойчивости будет способствовать выработке наиболее эффективных методов лечения и профилактики малярии.


