Масштаб распространенности ВИЧ-инфекции в России неуклонно растет. В 2018 г. в нашей стране число лиц, живущих с ВИЧ, превысило 1 млн человек1. Высокий уровень заболеваемости ВИЧ-инфекцией подчеркивает необходимость ранней и эффективной диагностики возбудителя. Однако для получения актуальной картины эпидемиологического процесса и определения уровня пораженности населения необходимо не только выявлять инфицированных лиц, но и определять число случаев инфицирования (заражения) за определенный период (например, за календарный год). Только этот параметр дает возможность определить реальную картину скорости распространения ВИЧ-инфекции среди населения [1, 2]. Фактически неопределенная доля выявляемых лиц может быть инфицирована задолго до момента обнаружения вируса, и их ВИЧ-статус станет известен только спустя какое-то время. Широко применяемые стандартные диагностические тест-системы не могут определить длительности заболевания, поэтому наблюдаемое увеличение или уменьшение числа пациентов с выявленной ВИЧ-инфекцией не обязательно отражает реальную картину эпидемического процесса. В связи с этим определение истинно новых случаев инфицирования для расчета показателя заболеваемости является на сегодняшний день одной из основных задач [3, 4].
Несмотря на то что выявление случаев недавнего заражения имеет решающее значение для оценки заболеваемости, все еще отсутствует «золотой» стандарт определения давности заражения ВИЧ [5]. Определение сроков инфицирования и возможность охарактеризовать инфекцию как недавнюю по-прежнему представляет собой проблему для врачей, эпидемиологов и сотрудников лабораторий, а трудности с определением даты заражения препятствуют не только оценке заболеваемости, но и надзору за распространением ВИЧ.
С 2009 г. ВОЗ и ЮНЭЙДС (UNAIDS) активно разрабатывают специализированные руководства для проведения исследований, направленных на выявление истинно новых случаев инфекции, а также проводят анализ эффективности применяемых тестов в разных странах [1–6].
Согласно руководствам ВОЗ, под недавней (ранней, свежей) инфекцией (recent infection) принято понимать период после инфицирования продолжительностью до 12 мес. Однако возможно изменение этой границы до 6–9 мес.: данный параметр зависит от используемых тестов и их характеристик. Давняя (поздняя, длительно текущая) инфекция (established/longstanding infection) начинает отсчет с отметки в 12 мес. или более раннего срока в зависимости от используемого подхода.
Необходимо отметить, что период ранней инфекции включает острую фазу (acute infection), ее называют также первичной инфекцией (primary infection). Данный период длится в среднем от 1 до 3 нед., от момента инфицирования и характеризуется выявлением только РНК ВИЧ и p24-антигена [7, 8]. С момента начала выявления антител к ВИЧ считают, что острая фаза заканчивается, и далее речь идет уже о недавней инфекции. Поэтому основным подходом к выявлению ранней инфекции является детекция сероконверсии – периода, когда начинается выработка антител к вирусным белкам.
Клиническое проявление ВИЧ-инфекции не позволяет распознать точное время заражения, поскольку на начальных этапах заболевание может протекать практически бессимптомно, или же неспецифические симптомы могут остаться незамеченными. Кроме того, острая ВИЧ-инфекция может значительно отличаться от типичного течения как по спектру симптомов, так и по тяжести клинических проявлений [9].
Образцовым форматом исследования для выявления случаев недавнего инфицирования являются продольные (longitudinal) исследования, которые характеризуются длительным анализом и наблюдением за пациентом в динамике, начиная с его ВИЧ-негативного статуса. Однако с технической точки зрения они требуют тщательного подбора пациентов, больших затрат и сложной ступенчатой методологии. Кроме того, исследования такого рода не являются репрезентативными для всех групп риска. Поэтому поперечные (cross-sectional) исследования стали хорошей альтернативой, где основной задачей выступает однократный анализ исследуемой выборки. Детальный анализ ВИЧ-специфичных биомаркеров недавней инфекции позволяет не только отойти от сложных продольных исследований, но и увеличить охват тестирования. Такой подход, как правило, дешевле, быстрее и легче реализовать на уровне популяции [4].
На сегодняшний день существует несколько различных направлений для выявления недавней инфекции: тесты, основанные на определении характеристик гуморального иммунного ответа, развивающегося после инфицирования; молекулярно-биологические тесты, позволяющие оценить давность заражения по анализу генома вируса; оценка клинической картины и лабораторных показателей, таких как вирусная нагрузка (ВН) и количество CD4-лимфоцитов. Однако ни один из этих способов не может дать во всех случаях качественную и достаточно точную оценку недавней инфекции. В то же время перечисленные подходы могут быть оптимизированы за счет объединения их в целостный алгоритм, который опирается на несколько лабораторных показателей, за счет чего повышается общая эффективность подхода [4, 10].
Необходимо помнить, что на точность и специфичность существующих методик негативно влияет вариабельность каждого индивидуального случая инфекции: скорость, сила и другие характеристики иммунного ответа; наличие оппортунистических инфекций, которые могут влиять на серологический профиль; уровень репликации вируса и возможное его подавление иммунной системой или в процессе лечения, а также субтип вируса. Эти факторы следует принимать во внимание при использовании существующих тест-систем и при разработке новых тестов, предназначенных для определения длительности ВИЧ-инфекции, поэтому объединение существующих методов в целостный алгоритм может в значительной степени решить эту проблему [4, 9, 11].
ВОЗ и ЮНЭЙДС уже разработали перечень рекомендаций по алгоритму выявления ранней ВИЧ-инфекции, который в зарубежной литературе известен как RITA (Recent Infection Testing Algorithm) [1–6]. Изначально применялся термин STARHS (Serologic Testing Algorithm for Recent HIV Seroconversion) – алгоритм, состоящий только из серологических тестов, направленных на анализ различных характеристик антител: концентрации, авидности, изотипа. В настоящее время STARHS заменен на RITA (также в литературе можно встретить термин MMA – Multi-Assay Approach – подход на основе нескольких тестов) [11], а алгоритм теперь включает в себя, помимо серологических тестов, ряд других исследований: тестирование на ВН, определение количества CD4-лимфоцитов, а также описание клинической картины. Данный перечень является базовым, но возможно его расширение за счет других тестов.
Добавление в состав алгоритма перечисленных исследований обусловлено тем, что применяемые серологические тесты могут привести к ошибочной классификации длительности инфекции. Например, такие группы пациентов, как непрогрессоры, лица, принимающие антиретровирусную терапию, и пациенты с прогрессирующим СПИДом не будут иметь стандартный серологический профиль, поэтому интерпретация результатов на основе серологических тестов может быть искажена. Для адекватной оценки таких случаев необходим комплексный подход. Поэтому определение показателя ВН, количества CD4-лимфоцитов и учет клинической картины позволяют корректировать результаты. Так, при анализе ВН необходимо понимать, что высокая концентрация вируса в крови может свидетельствовать о первичной инфекции, а недетектируемый ее уровень может быть вызван как приемом антиретровирусной терапии, так и естественным подавлением уровня репликации вируса в случае тестирования непрогрессора. Определение высоких уровней CD4-лимфоцитов позволяет выявлять раннюю инфекцию и исключать пациентов в стадии СПИДа, когда количество лимфоцитов минимально. Описание клинической картины также может позволить исключить неправильную классификацию, если есть информация по СПИД-индикаторным заболеваниям, что в свою очередь будет свидетельствовать о давней инфекции [12].
Как и любая система, алгоритм требует предварительной валидации и калибровки на части той выборки, которая в дальнейшем будет подвергнута анализу. Для этого специалистами ВОЗ были выделены 2 параметра, требующие расчета перед началом использования алгоритма [1–6]. Определение этих параметров необходимо в связи с тем, что анализ может привести к ошибочной классификации пациентов с длительной, в частности, бессимптомной ВИЧ-инфекцией как недавно инфицированных. Первый показатель – средняя продолжительность ранней инфекции (MDRI – mean duration of recent infection) – определяется как время, прошедшее с момента заражения до момента, когда инфекцию можно охарактеризовать как недавнюю (от 6 до 12 мес.). Этот параметр позволяет определить временну́ю границу, относительно которой тест-система или алгоритм будут классифицировать инфекцию как раннюю или позднюю. Второй показатель – уровень ложно ранних результатов (FRR – false recent ratio) – это доля давних случаев инфицирования, которая ошибочно классифицируется тестом как «недавняя инфекция». Чем ниже данный показатель, тем выше специфичность применяемого теста или алгоритма. Так, с 2016 г. в Ирландии в систему эпидемиологического надзора за ВИЧ-инфекцией был включен такой алгоритм для выявления недавней инфекции: показатель FRR составляет 1,3%, а MDRI равен 188 дням, то есть все образцы, которые на основании используемых тестов выявляются как ранние, предположительно имеют длительность инфекции менее 188 дней, при этом только 1,3% образцов с давней инфекцией ошибочно определяются как ранние [13].
Необходимо отметить, что подобные алгоритмы и тесты преимущественно разрабатываются и применяются в Европе и США, где преобладает ВИЧ-1 субтипа В. Поэтому и разработчики тестов, и специалисты ВОЗ делают акцент на том, что данные методы должны пройти предварительное тестирование на хорошо охарактеризованной выборке – образцах с известными сроками заражения, а этап валидации на других субтипах ВИЧ остается главным ограничением в использовании уже существующих методик. Особое внимание необходимо уделять пациентам, которые уже получают терапию в момент тестирования, а также непрогрессорам, лабораторные показатели которых могут снизить точность анализа и привести к ошибочной классификации, увеличивая FRR применяемой методики [6].
Следует учитывать и тот факт, что практически все существующие тесты, в частности, серологические, разработаны на основе коммерческих тест-систем и представлены модифицированными стандартными протоколами. В целом такие тест-системы потенциально экономически эффективны, однако основным препятствием для внедрения является возможное отсутствие их на рынке.
Серологические тесты для выявления недавней ВИЧ-инфекции
Первые месяцы после заражения ВИЧ являются самыми важными, поскольку имеется возможность регистрации периода, когда начинается выработка специфических маркеров. Первыми из них в крови появляются РНК и провирусная ДНК ВИЧ, которые могут быть выявлены приблизительно с 10–14-го дня после заражения. С 16–18-го дня возможна детекция р24-антигена, а спустя 3–4 нед. – выявление антител к ВИЧ (см. рисунок) [7, 8].
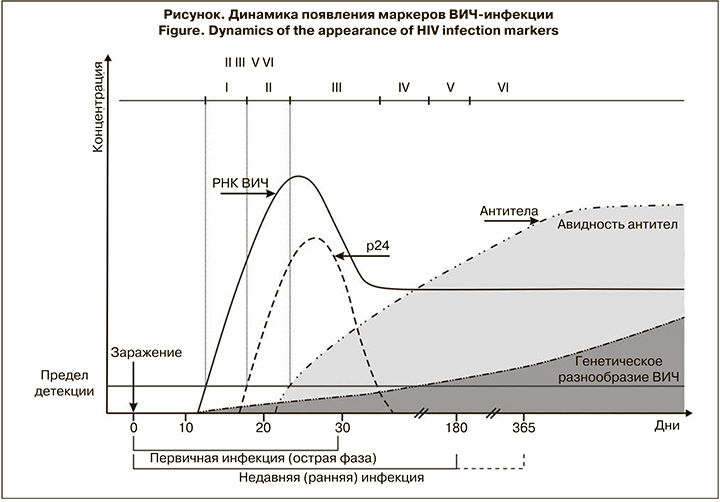
В течение 1-го месяца после инфицирования титр антител обычно невысок, также как их аффинность (степень сродства с антигеном) и авидность (прочность связей в комплексе антиген–антитело). Поэтому увеличение титра и специфичности антител может свидетельствовать о недавнем заражении. Эти принципы легли в основу серологических тестов, позволяющих оценивать давность инфицирования ВИЧ [9, 11].
ИФА с заниженной чувствительностью (Detuned assay)
Данный подход является первым, он лег в основу тестов для выявления ранней ВИЧ-инфекции. Он был разработан и описан в 1998 г. R.S. Janssen и соавт. [14] и назывался «sensitive/less sensitive enzyme immunoassay» (S/LS-EIA) – ИФА с заниженной чувствительностью, или «расстроенный» ИФА (detuned assay). Снижение чувствительности теста достигается модификацией исходной тест-системы путем разведения анализируемого образца или сокращением времени инкубации, при этом образцы тестируются в 2 повторах: по стандартному и модифицированному протоколам. Принцип работы основан на том, что у недавно инфицированных уровень антител к ВИЧ будет ниже по сравнению с теми, у кого заражение произошло давно. Недавняя инфекция идентифицируется, когда образец дает положительный результат в исходном протоколе, но отрицательный в модифицированном, поскольку после разведения образца концентрация антител снижается до недетектируемого уровня. У лиц с длительно текущей инфекцией, а значит, с высоким титром антител, будут получены положительные результаты в обоих протоколах.
Первоначально такой подход был разработан на основе тест-системы первого поколения Abbott HIV-1 3A11 (Abbott Laboratories, США). Такие тест-системы, как Vironostika HIV-1 MicroElisa (bioMerieux, Франция) [на сегодняшний день не выпускается, на смену ей пришла тест-система Avioq HIV-1 MicroElisa system (Avioq Inc., США)], Determine HIV-1/2 assay (Abbott Laboratories, США), OraQuick Advance HIV-1/2 assay (OraSure Technologies, Inc., США), Uni-Gold Recombigen (Trinity Biotech, Ирландия), HIV-1/2 particle agglutination test (Serodia, США) также были модифицированы по этому принципу. Порог определения ранней инфекции для теста Abbott 3A11 составляет 129 дней, а для Vironostika-1 MicroElisa – 170 дней [9].
BED-тест
В зарубежной литературе тест называется BED-CEIA (BED capture enzyme immunoassay). Это название он получил по названиям субтипов ВИЧ-1, на которые направлен: для выявления антител используется пептид, содержащий иммунодоминантные области трансмембранного белка gp41 подтипа B, рекомбинантной формы CRF_01AE (ранее подтип E) и подтипа D [10]. Метод представляет собой количественный ИФА, основанный на определении соотношения специфического IgG к ВИЧ к общему IgG в образце. Как правило, это соотношение ниже на ранних сроках после инфицирования, поскольку доля ВИЧ-специфического IgG к общему IgG увеличивается в течение первых 2 лет после заражения.
Одним из преимуществ теста является то, что для упрощения сбора, транспортировки и хранения образцов, а также для расширения использования BED-теста была разработана процедура подготовки пятен крови, плазмы и сыворотки, высушенных на фильтровальной бумаге. Единственным представителем данного направления является BED EIA HIV-1 Incidence Test (Calypte Biomedical Corporation, США), у которого граница выявления ранней инфекции составляет 155 дней [9].
Определение авидности антител (avidity assay protocol)
Авидность – характеристика общей стабильности комплекса антиген–антитело – определяется как индекс авидности (ИА; AI – avidity index), который рассчитывается как соотношение сигналов, полученных для двух аликвот одного образца: одна разводится диссоциативным (хаотропным) агентом, вторая – солевым или промывочным буфером. В качестве диссоциативного агента могут выступать гуанидин гидрохлорид, тиоцианат калия, диэтиламин или мочевина. Они нарушают водородные связи и мешают взаимодействию антигена с антителом. Установлено, что на ранних сроках инфекции антитела обладают низкой авидностью, тогда как зрелые антитела на поздних сроках имеют высокий уровень авидности и устойчивы к воздействию диссоциативного агента, образуя крепкую связь с антигеном.
Одной из первых была модифицирована коммерческая тест-система 3-го поколения HIV-1/2 gO EIA (Abbott Diagnostics, США), однако сейчас на смену ей пришла ARCHITECT HIV Ag/Ab Combo (Abbott Diagnostics, США) [15]. Граница выявления ранней инфекции равна 180 дням. В данном сегменте также известны тест-системы HIV-1 Limiting Antigen Avidity EIA (LAg) (Sedia Biosciences, США), Anti-HIV-1/2 Vitros ECi assay (Ortho-Clinical Diagnostics, США) и Genetic Systems HIV-1/2 Peptide EIA (Bio-Rad Laboratories, США). Установлено, что тесты на определение индекса авидности являются более чувствительными, чем BED-тест и ИФА с заниженной чувствительностью [16].
Анализ IDE-V3
В основу названия теста легли аббревиатуры иммунодоминантного эпитопа трансмембранного гликопротеина gp41 (Immunodominant Epitope gp41) и V3-петли гликопротеина gp120 (gp120-V3 loop). Разведения каждого образца тестируются в двух повторах: антитела против IDE и V3 детектируются отдельно. При этом антитела против IDE нарабатываются быстрее, чем антитела против V3, а реакционная способность антител выше у людей с длительно текущей инфекцией [17]. Граница выявления ранней инфекции равна 180 дням.
Анализ IDE-V3 не является коммерческим продуктом, поэтому любая профильная лаборатория может применять этот тест, закупив все необходимые расходные материалы и реактивы. Он был специально разработан для обеспечения непрерывной доступности теста, независимо от любого коммерческого источника. IDE-V3 используется для мониторинга и расчета оценки заболеваемости во Франции с 2003 г. [9].
Определение IgG3 антител против p24 (p24 IgG3 assay)
Метод основан на выявлении антител изотипа IgG3 против p24. Было показано, что эти антитела появляются на ранней стадии и обнаруживаются только в первые 4 мес. после инфицирования, что делает их потенциальным маркером недавней ВИЧ-инфекции [18]. Таким образом, на основании этого признака достоверно можно идентифицировать образцы «ранних» сывороток, полученных в период от 2 до 4 мес. после заражения, однако весь период ранней инфекции (до 12 мес.) тест не охватывает. Среди коммерческих тест-систем на рынке присутствует HIV-1 Bio-Plex asssy (Bio-Rad Laboratories, США).
Анализ INNO-LIA HIV-1/2
Знание того, что титр антител повышается после периода сероконверсии, и антитела, направленные против различных вирусных белков, появляются в разное время, был разработан тест INNO-LIA HIV-1/2 (Innogenetics, Бельгия). Анализ был первоначально разработан для подтверждения диагноза и представляет собой иммуноблот: он обнаруживает антитела к рекомбинантным пептидам ВИЧ-1 (p17, p24, p31, gp41 и gp120) и ВИЧ-2 (gp36 и gp105). Интенсивность полос оценивают и используют для определения давности инфекции. Поскольку нет необходимости в изменении процедуры анализа, он может одновременно служить как подтверждающим диагностическим тестом, так и тестом на недавнюю инфекцию, тем самым позволяя выявлять последнюю без каких-либо дополнительных затрат, но это выгодно в тех странах, где обычно используют INNO-LIA HIV-1/2 для подтверждения диагноза ВИЧ. Однако ограничением для использования этого теста является то, что его можно провести в течение 36–67 дней с момента начала детекции антител [11].
Тест-система ДС-ИФА-ВИЧ-АТ-СРОК
Единственным отечественным разработчиком теста для определения длительности ВИЧ-инфекции является ООО «НПО «Диагностические Системы» (Нижний Новгород, Россия). Данный тест является усовершенствованным вариантом «расстроенных» тестов (detuned assay). Принцип его работы также основан на различиях в титре антител к ВИЧ у недавно и длительно инфицированных людей. В то же время по дизайну тест больше напоминает тест на авидность: в анализе участвуют 2 аликвоты – исходный образец и его разведение, только вместо хаотропного агента для разведения исходного образца используется солевой буфер. Принадлежность образца к «ранним» или «поздним» оценивается по проценту падения оптической плотности (ОП) разведенного образца по отношению к неразведенному. Тест-система ДС-ИФА-ВИЧ-АТ-СРОК позволяет дифференцировать недавнюю (до 9 мес.) и длительно текущую ВИЧ-инфекцию с точностью 95,02% (91,81–97,01) [18].
Методы, основанные на анализе вирусного генома
Молекулярные методы, позволяющие исследовать геном вируса, в настоящее время становятся перспективным подходом к определению длительности инфекции. Основой этого направления является особенность увеличения генетического разнообразия (вариабельности) вируса в процессе болезни, детекцию которого можно провести разными методами [9, 11, 19].
Анализ температуры плавления ампликонов (HMA – High Resolution Melting Assay) – один из самых простых молекулярных методов. Он заключается в анализе температуры плавления дуплексов ДНК, полученных в ходе ПЦР. Наиболее информативными являются регионы gag, pol и env [11]. Было показано, что увеличение температуры плавления свидетельствует об увеличении генетического разнообразия вируса, что в свою очередь может указывать на давние сроки инфицирования. Основными недостатками данного метода являются его чувствительность к вставкам и делециям, которые достаточно часто встречаются в геноме ВИЧ, и неспособность различать случаи, вызванные несколькими генетическими вариантами ВИЧ. С этими задачами хорошо справляются методы, основанные на секвенировании. При анализе генома или его фрагментов анализу и подсчету подвергаются вариабельные позиции – те позиции, в которых произошла детекция более 1 нуклеотида. На ранних стадиях инфекции их количество минимально, затем этот показатель увеличивается, что может свидетельствовать о длительно текущей инфекции [19, 20].
Заключение
Тесты для дифференциальной диагностики ранней ВИЧ-инфекции существуют и широко доступны. Определение длительности инфекции на основании одного биомаркера является недостоверным подходом, поскольку адекватная оценка затруднена сложной и нелинейной динамикой иммунного ответа. Поскольку каждый из существующих методов имеет свои недостатки, наиболее достоверная информация может быть получена путем одновременного применения 2 и более тестов, объединенных в единый алгоритм. Сочетание серологических и молекулярных методов может обеспечить хороший результат при выявлении недавней ВИЧ-инфекции.
На сегодняшний день во многих зарубежных странах уже есть опыт применения перечисленных подходов и тестов для выявления недавней ВИЧ-инфекции как в отдельности, так и в составе целостных алгоритмов. Однако такие исследования преимущественно имеют статус пилотных с целью определения аналитических характеристик используемых тестов, способов расчета уровня заболеваемости и описания анализируемой выборки.
Результаты таких работ позволяют вести разработку протоколов для дальнейшего применения существующих подходов и их внедрения в практику рутинного эпидемиологического надзора. ВОЗ и ЮНЭЙДС призывают к активной работе по совершенствованию алгоритмов диагностики ВИЧ для снижения и остановки распространения вируса, поэтому вопрос определения длительности инфекции является приоритетным и занимает важное место в ряду исследований. Еще одно преимущество выявления лиц на ранних стадиях инфекции связано с возможностью раннего назначения антиретровирусной терапии. Ранняя диагностика может также способствовать детальному описанию тех вариантов вируса, которые циркулируют и передаются в популяции в конкретный период времени: определение генотипа и профиля резистентности вируса являются основными характеристиками и вносят большой вклад в анализ эпидемиологической картины распространения ВИЧ.


