В настоящее время накоплено большое количество данных об использовании двухкомпонентных режимов АРТ в качестве начального или поддерживающего режима лечения больных ВИЧ-инфекцией. Преимущества упрощения заключаются в сокращении количества таблеток, исключении возможного негативного влияния третьего препарата [как правило, тенофовира (TDF) или абакавира (АВС)] и снижении стоимости лечения.
В ранних исследованиях оценивали сочетание эффективного, хорошо переносимого нуклеозидного ингибитора обратной транскриптазы (НИОТ) ламивудина (3TC) с усиленными ритонавиром ингибиторами протеазы (ИП/р) [1–3]. Однако применение ИП/р ассоциировано с ранними и поздними метаболическими нарушениями и межлекарственными взаимодействиями, а ненуклеозидные ингибиторы обратной транскриптазы (ННИОТ) 1-го поколения имеют низкий генетический барьер резистентности, что ограничивает их привлекательность в качестве схем двухкомпонетной терапии [4].
Ингибитор интегразы (ИИ) долутегравир (DTG) стал подходящим кандидатом для включения в двухкомпонентную схему АРТ в сочетании с 3TC, поскольку имеет высокую эффективность, высокий барьер для развития резистентности, вызывает минимум побочных эффектов и принимается 1 раз в сутки [5–7].
В исследованиях GEMINI 1 и 2 у пациентов с ВИЧ-1, не получавших ранее АРТ, двухкомпонентная схема обеспечила быструю, устойчивую вирусологическую супрессию, независимую от исходных характеристик пациента, а также иммунологический ответ, сравнимый с таковым при применении стандартной трехкомпонентной схемы АРТ в конечных точках на 48-й, 96-й и 144-й нед. [8, 9].
Переключение пациентов со стабильной трехкомпонентной схемы на двухкомпонентную DTG + 3TC (продолжающееся исследование TANGO) также показало не меньшую эффективность этого режима [10].
Для пациентов, начинающих лечение, сочетание DTG + 3TC одобрено американскими и европейскими рекомендациями для большинcтва людей, живущих с ВИЧ. Дополнительные условия – исходный иммунный статус не менее 200 клеток/мкл (только в рекомендациях International Antiviral Society-USA, 2020), ВН не более 500 000 копий/мл, отсутствие ВГВ или результатов теста на резистентность к 3TC (в американских рекомендациях DHHS, 2021) [11–13]. В отечественных клинических протоколах данный режим пока включен в первую линию только для пациентов старше 50 лет или имеющих нарушения липидного и углеводного обмена, а также больных с риском сердечно-сосудистой патологии как альтернативный режим, а для пациентов с удачным опытом АРТ рекомендован как вариант для оптимизации [14].
Специалисты признают необходимость более длительного и масштабного изучения применения двухкомпонентной схемы в широкой популяции пациентов. Представляем результаты собственного рандомизированного открытого контролируемого исследования по применению DTG + 3TC в сравнении со стандартными трехкомпонентной АРТ.
Целью исследования была оценка эффективности и безопасности переключения больных ВИЧ-инфекцией, ранее получавших стандартные трехкомпонентные схемы АРТ, на двухкомпонентную схему, включавшую DTG и 3ТС, в течение 96 нед.
Материалы и методы
Отбор пациентов проходил в СНИОЭП СПИД Центрального НИИ эпидемиологиии Роспотребнадзора с января 2019 г. по ноябрь 2020 г. В исследование был включен 231 больной ВИЧ-инфекцией с опытом лечения. Пациентов рандомизировали в 2 группы: больных 1-й группы (100 чел.) переключали на двухкомпонентную схему АРТ (DTG + 3ТС), а пациенты 2-й группы (группа сравнения, 131 чел.) продолжили получать текущую стандартную схему АРТ. В настоящее время исследование продолжается. Исходные характеристики больных представлены в табл. 1.

Группы были сопоставимы по полу, возрасту, путям инфицирования, стадиям ВИЧ-инфекции, исходному иммунному статусу, длительности предыдущей АРТ. Соотношение мужчин и женщин в обеих группах было 2:1 с преобладанием мужчин. Возраст пациентов достаточно сильно колебался (от 25 до 70 лет), но различий медианы возраста в группах не было. Более 50% пациентов инфицировались гетеросексуальным путем.
Около 90% пациентов находились на стадиях ВИЧ-инфекции 3 и 4А. Среди вторичных заболеваний преобладали кандидоз слизистых оболочек ротовой полости, герпетическая инфекция (Herpes simplex, Herpes zoster) и туберкулез. У всех пациентов вторичные заболевания были в анамнезе. Сопутствующими заболеваниями страдали 53 и 47,3% больных 1-й и 2-й групп соответственно. Антитела к ВГС имели 18–26,5% пациентов, но РНК ВГС была обнаружена лишь у 4 (1,7%). Курение сигарет отметили 25 и 39,7% больных соответственно. Медиана длительности АРТ в 1-й и 2-й группах не отличалась и составила 6–8 лет (от 1 года до 19 лет). Более чем у половины пациентов до начала исследования схема АРТ была изменена минимум 1 раз (66 и 51,9% в 1-й и 2-й группах соответственно). Доля больных, у которых уже использовали 4 и более схем АРТ, составила 23 и 5,3%. На двухкомпонентный режим со схем, включавших ИП/р, переключали 35% пациентов, ННИОТ – 11%, другие ИИ – 54% (из них 83,3% получали DTG в трехкомпонентной схеме). До переключения в 1-й группе 56% пациентов принимали TDF, 27% – АВС, остальные 10% – зидовудин (ZDV), фосфазид (Ф-AЗТ) или диданозин (ddI). Во 2-й группе 33,6% больных продолжили получать ИП/р [лопинавир (LPV), атазанавир (ATV), дарунавир (DRV)], 64,9% – ННИОТ [эфавиренз (EFV) , невирапин (NVP), рилпивирин (RPV)], 1,5% – ИИ [ралтегравир (RAL)]. Большинство пациентов (72,5%) остались на схеме с TDF (или были переключены на этот препарат с другого НИОТ), остальным были назначены АВС (25,2%) или Ф-AЗТ (1,5%).
В соответствии с критериями включения все пациенты имели неопределяемую ВН в течение последних 6 мес., количество CD4+-лимфоцитов, превышающее 200 клеток/мкл, и не имели маркеров гепатита В. Медианы абсолютного и относительного количества CD4+-лимфоцитов достоверно не отличались в обеих группах и составляли 627 (31%) и 607,5 клеток/мкл (32%).
В исследовании применяли MITT-анализ (модифицированный анализ – Modified Intention-to-treat), предусматривающий включение всех пациентов, получивших хотя бы 1 дозу препаратов и имеющих хотя бы 1 исследование ВИЧ РНК после визита на неделе «0».
До начала исследования, через 48 и 96 нед. методом проточной цитометрии определяли количество CD4+- и CD8+-лимфоцитов, а методом ПЦР – уровень РНК ВИЧ (чувствительность теста – 50 копий/мл).
Безопасность схемы АРТ оценивали по частоте развития нежелательных явлений (НЯ) различной степени тяжести по данным субъективных жалоб, физикального осмотра, жизненных показателей, лабораторных исследований. Параметры анализа периферической крови и биохимического анализа крови исследовали до лечения, через 48 и 96 нед. терапии.
Внутригрупповые изменения параметра оценивали с помощью t-теста Стьюдента для нормально распределенных данных или знакового критерия Вилкоксона (Манн–Уитни) для данных, не имеющих нормального распределения. В качестве теста на нормальность распределения применяли тест Шапиро–Вилка. Использовали компьютерную программу Biostat.
Результаты и обсуждение
На 01.06.2021 закончили 48 нед. исследования 86 и 111 пациентов, 96 нед. – 35 и 40 пациентов 1-й и 2-й групп соответственно. У подавляющего большинства больных концентрация РНК ВИЧ была ниже порога детекции (< 50 копий/мл). Лишь у 4 больных (по 2 из каждой группы, 2,3 и 1,8% соответственно) на 48-й нед. наблюдали вирусологический всплеск, не превышавший 246 копий/мл. Вирусологическим всплеском считали кратковременное повышение уровня РНК ВИЧ выше порога чувствительности тест-системы, но не более 400 копий/мл [11]. Таким образом, в течение 96 нед. лечения вирусологической неэффективности терапии отмечено не было, что согласуется с данными других исследователей [9].
В обеих группах наблюдали достоверный прирост количества CD4+-лимфоцитов (p < 0,01). Медиана количества CD4+-лимфоцитов к 48-й нед. увеличилась на 66,5 и 28,5 клеток/мкл относительно исходного уровня в 1-й и 2-й группах, к 96-й нед. – соответственно на 66 и 39,5 клеток/мкл (рис. 1).
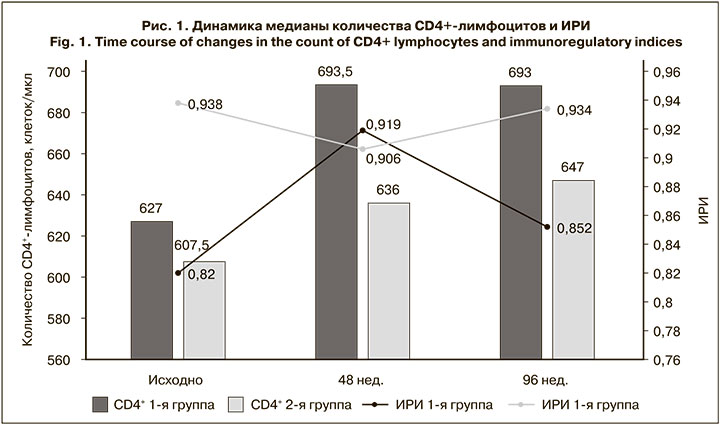
Иммунологическая эффективность в 1-й группе была достоверно выше как на 48-й, так и на 96-й нед. лечения (p < 0,05). Медиана иммунорегуляторного индекса (ИРИ – соотношение CD4+/CD8+) во 2-й группе пациентов практически не изменилась, в то время как у пациентов 1-й группы к 96-й нед. этот показатель несколько увеличился. Доля больных с исходным количеством CD4+-лимфоцитов < 350 клеток/мкл в 1-й группе сократилась с 10 до 2,9%, во 2-й – с 14,5 до 2,5%. В процессе исследования клинических и лабораторных симптомов вторичных заболеваний не было зафиксировано ни у одного из пациентов, что свидетельствовало об отсутствии клинической прогрессии ВИЧ-инфекции.
Приверженность лечению в исследуемых группах была высокой. На 48-й нед. более 95% назначенных доз препаратов принимали 97,7 и 98,2% больных, на 96-й нед. – все больные.
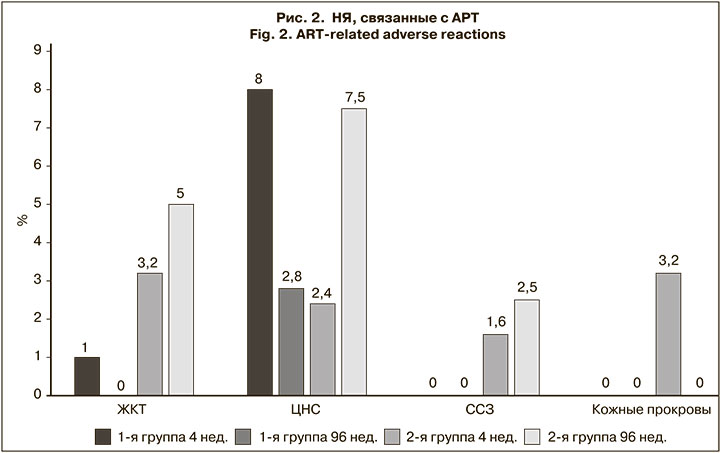
Переносимость АРТ была достаточно хорошей в обеих группах Большинство НЯ не были связаны с АРТ, имели легкую степень тяжести и не требовали лекарственной коррекции. В 1-й мес. лечения у 9–10% пациентов в каждой группе зарегистрировали клинически выраженные НЯ (рис. 2). У пациентов 1-й группы отмечали в основном нарушения со стороны ЦНС (плохой сон, головная боль, головокружение), у пациентов 2-й группы НЯ были обусловлены конкретными препаратами (нарушения со стороны желудочно-кишечного тракта при приеме LPV/r, патология ЦНС и сыпь при приеме EFV, пожелтение склер при приеме ATV). Через 96 нед. 1 (2,8%) пациент в 1-й группе продолжал жаловаться на плохой сон, в то время как доля пациентов с НЯ во 2-й группе возросла с 10,4 до 15%. Так, 5% больных предъявляли жалобы на чувство тошноты и диарею после приема LPV/r; 7,5% – на инверсию сна, раздражительность, периодическую головную боль и головокружение после сна, связанные с приемом EFV, а 5% – на сердечно-сосудистую патологию. В связи с указанными НЯ (6,9%), а также по причине упрощения (1,5%) пациентам 2-й группы произвели замены АРВ-препаратов: к 48-й нед. исследования 7 пациентам (на RPV – 3, на элсульфавирин (ESV) – 3, на RAL – 1), на 96-й нед. – еще 4 (на RPV – 2, на ESV – 1, на RAL – 1). У всех пациентов изменение схемы АРТ не отразилась на ее эффективности (РНК ВИЧ < 50 копий/мл), но в дальнейшем анализе они не учитывались. Кроме того, из 2-й группы к 48-й нед. выбыли еще 5 пациентов (трое уехали по месту жительства, 1 – в длительную командировку, 1 пациентка – по причине беременности). В 1-й группе, замен препаратов не было, 2 пациента умерли на 4-й и 12-й нед. исследования в связи с выявленной злокачественной опухолью (рак ободочной кишки и рак поджелудочной железы).
Исходно у 2–3,8% пациентов регистрировали снижение уровня Hb < 100 г/л, но к 96-й нед. эти показатели у всех больных пришли в норму. Остальные показатели общего анализа крови не претерпели изменений в обеих группах.
Основные биохимические показатели существенно не изменялись на фоне терапии (табл. 2). Медиана уровней глюкозы, билирубина, креатинина, АЛТ и АСТ у подавляющего большинства пациентов оставалась в пределах нормы. В обеих группах мы не наблюдали также различий в доле больных с отклонениями этих показателей в процессе лечения по сравнению с исходными значениями.
Вместе с тем, в 1-й группе мы зарегистрировали уменьшение доли пациентов со снижением скорости клубочковой фильтрации (СКФ) ниже 90 мл/мин с исходных 16% до 8,6% на 96-й нед. исследования (р < 0,01), что вероятно, связано с отсутствием влияния TDF у 56% больных, ранее его получавших. Среди пациентов 2-й группы этот показатель не изменился и сохранялся на уровне 5–7%. Снижения СКФ ниже 60 мл/мин не наблюдали ни у одного пациента.
Существенно различались в группах доли больных с повышением уровня щелочной фосфатазы (ЩФ). Так, медиана ЩФ в 1-й группе уменьшилась с 75 до 61 Ед/л, а во 2-й группе не изменилась. Если исходно доля таких пациентов в 1-й группе составила 8%, то к 96-й нед терапии этот параметр нормализовался у всех пациентов (р < 0,01). У пациентов 2-й группы, напротив, повышение уровня ЩФ выявляли в 2 раза чаще по сравнению с исходным показателем (15%; р < 0,01), что связывали с сочетанным влиянием 2 факторов – возрастными особенностями и действием TDF. Так 66,7% пациентов с превышенным уровнем ЩФ были старше 45 лет и принимали TDF в схеме АРТ, но взаимосвязь статистически не прослеживалась из-за небольшого числа пациентов, закончивших 96 нед. терапии. Для подтверждения выявленного факта необходимы дальнейшие исследования.
Была отмечена тенденция к уменьшению доли пациентов с повышенным уровнем ГГТ в 1-й группе, но разница не была достоверна, в то время как среди больных 2-й группы этот показатель достоверно возрос по сравнению с исходным с 33,6 до 40% (р < 0,05). Медиана уровня ГГТ также увеличилась с 31 до 41 Ед/л.
Изменения липидного профиля наблюдали преимущественно во 2-й группе (табл. 3).
Так, число больных с повышением уровня общего холестерина (ОХ) увеличилось через 96 нед. на 23% (р < 0,01) в основном за счет фракции липопротеидов низкой плотности (ЛПНП), повышение которой регистрировали у 67,5% пациентов. Однако из-за увеличения медианы липопротеидов высокой плотности (ЛПВП) индекс атерогенности не только не изменился, но даже несколько уменьшился.
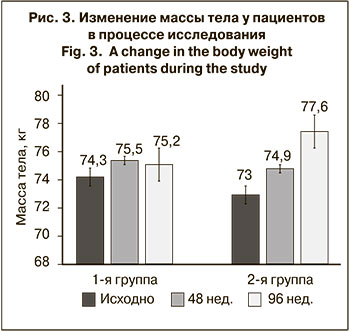
Треть больных 2-й группы к концу исследования имела также достоверное повышение уровня триглицеридов (ТГ) (р < 0,01). Двухкомпонентная схема оказалась более толерантной к изменениям липидных параметров. В 1-й группе увеличилась доля больных, имевших повышенный уровень ЛПНП, и уменьшилась доля пациентов со снижением уровня ЛПВП, но медианы этих показателей остались прежними. Масса тела (по медиане) возросла за 96 нед. в 1-й группе всего на 0,9 кг, тогда как во 2-й группе – на 4,6 кг (рис. 3).
Заключение
Схема двухкомпонентной терапии DTG + 3TC, применяемая у пациентов с предшествующим опытом стабильного лечения, показала такую же высокую эффективность, как и стандартная терапия, включающая 3 препарата. К 96-й нед. 100% пациентов в нашем исследовании имели ВН ниже порога чувствительности тест-системы (< 50 копий/мл). В процессе лечения вирусологической неэффективности не зарегистрировано. У всех пациентов, достигших точки 96 нед., отмечали высокую приверженность терапии (> 95%).
Клинически выраженные НЯ, связанные с АРВ-препаратами, зафиксировали в конечной точке исследования на 96-й нед. у 2,8% пациентов 1-й группы и у 15% пациентов 2-й группы. Во всех случаях побочные эффекты были легкой степени тяжести и не требовали медикаментозного лечения, однако явились причиной модификации схемы стандартной терапии у 6,9% больных.
Незначительные лабораторные отклонения основных биохимических показателей наблюдали в обеих группах с одинаковой частотой. Вместе с тем, при переходе на двухкомпонентную схему АРТ зарегистрировали отсутствие существенного влияния на липидный профиль, достоверное снижение доли пациентов со СКФ < 90 мл/мин в 2 раза и нормализацию уровня ЩФ у всех больных. За 96 нед. лечения медиана веса пациентов возросла всего на 0,9 кг (изменения не были достоверными).
Таким образом, упрощенная схема, состоящая из DTG и 3TC, так же эффективна у пациентов с опытом терапии, как и стандартные схемы АРТ, но существенно превосходит их по переносимости и безопасности.


