В 2020 г. мир столкнулся с новой, ранее не изученной коронавирусной инфекцией. По данным ВОЗ (на момент написания статьи), в мире коронавирусом нового типа инфицировано более 3 млн человек, погибли более 170 000 . Большая часть летальных исходов связана с формированием специфической коронавирусной пневмонии. Предположительно, основной источник формирования тяжелой гипоксии зашифрован в гемоглобинопатии, вызываемой вирусом. Существенную роль играют нарушения кровотока. На наш взгляд, это лишь одно из звеньев патогенеза [1, 2].
COVID-19 (от англ. Corona Virus Disease 2019) – бета-коронавирус, схожий по структуре с тяжелым респираторным синдромом (SARS): оба вируса имеют белок, взаимодействующий с рецепторами ангиотензин-превращающего фермента 2 (АПФ-2), что обеспечивает проникновение в клетку. По данным филогенетического анализа 103 штаммов вируса были идентифицированы 2 типа SARS-CoV-2: L и S. Тип L преобладал в первые дни эпидемии в Китае. Однако за пределами Уханя этот штамм циркулировал реже [2]. Первоначальный природный резервуар коронавируса нового типа был ассоциирован с летучей мышью, часто употребляемой жителями КНР в пищу. Однако позже была подтверждена способность вируса передаваться от человека человеку сначала воздушно-капельным, затем контактным путями. Не исключен также фекально-оральный механизм передачи. В большинстве случаев длительность инкубационного периода от момента инфицирования до появления первых симптомов составляет от 1 до 14 дней, но, по некоторым наблюдениям, может длиться до 3 нед. и более. Симптомы дебюта COVID-19 практически не позволяют отличить эту инфекцию от других ОРВИ.
После попадания на слизистые оболочки респираторного тракта клетки вируса взаимодействуют с дыхательным эпителием посредством АПФ-2. Проникая в клетку, вирус разрывает свои шипы (формирующие корону и представленные специфическими гликанами), высвобождая токсический белок, который, устремляясь в нижележащие дыхательные пути, вызывает специфические изменения в виде очагов фиброза, снижения эластичности альвеолярного аппарата, а также диффузного поражения легочной ткани [3, 4]. Кроме того, существует мнение, что новая коронавирусная инфекция способна нарушать синтез гема, связываясь при помощи специфических белков с рецепторами CD147, находящимися на поверхности эритроцитов, что вызывает фактически кислородное голодание [5, 6]. Такая точка зрения объясняет эффективность применения препаратов группы хлорохинов. По своим специфическим свойствам они могут предотвращать атаку вирусных белков на синтез гема. По степени тяжести различают бессимптомное носительство, легкую, среднюю и тяжелую формы заболевания. В ряде источников отмечено, что даже при бессимптомном носительстве, после прекращения вирусовыделения на компьютерной томографии (КТ) легких у некоторых пациентов визуализируются очаги фиброза и изменения легочной ткани по типу «матового стекла» [7–8].
Цель исследования – формулирование гипотезы патогенеза СOVID-19 и разработка алгоритма патогенетической терапии СOVID-19 по степени тяжести заболевания.
Материалы и методы
Проанализировано более 30 литературных источников, содержащих данные о течении, диагностике и терапии COVID-19 [Pubmed, Scopus, Библиотека ВОЗ], а также клинические примеры из собственной практики Опубликованные результаты наблюдений за течением и особенностями ответа на проводимую терапию свидетельствуют о значительной частоте негативных реакций у пациентов. Отмечено повышение риска летального исхода при применении глюкокортикостероидов [3, 5, 9]. В части исследований выявлено негативное влияние некоторых нестероидных противововоспалительных препаратов (НПВП): у больных COVID-19 их прием сопровождался увеличением риска тромбозов и нежелательного усиления иммунного ответа [10]. Хлорохин и гидроксихлорохин, которые, по первоначальным данным, ингибируют SARS-CoV-2 in vitro [11], были впервые включены в руководства по лечению Национальной комиссии здравоохранения Китая. Данные первых клинических рандомизированных исследований продемонстрировали преимущества их назначения пациентам с COVID-19 [12, 13]. Использование у пациентов с COVID-19 гидроксихлорохина (200 мг 3 раза в день в течение 10 дней) сопровождалось более высокой частотой элиминации SARS-CoV-2 в образцах из носоглотки на 6-й день (в 70% случаев), тогда как при отсутствии препарата или другой этиотропной терапии элиминация была подтверждена лишь в 12,5% случаев [14].
Исследование препарата лопинавир/ритонавир, используемого в качестве ингибитора протеаз при ВИЧ-инфекции, показало, что он обладает активностью против SARS-CoV в исследованиях на животных [15, 16]. Однако в рандомизированном исследовании 199 пациентов с тяжелым COVID-19 по сравнению с теми, кто получал базисную терапию, достоверной разницы не выявлено [17].
Результаты анализа течения и исходов коронавирусной инфекции у пациентов с тяжелой формой указывают на ряд особенностей заболевания:
- ухудшение состояния с развитием выраженной одышки нередко возникает внезапно [18–23];
- поражения легких, по данным КТ, проявляются характерными для вирусных пневмоний и встречающимися при ряде других патологических процессов (туберкулезе, онкологической патологии) изменениями по типу «матового стекла»;
- патоморфологические изменения легких напоминают картину гемической гипоксии, развивающейся при отравлениях метгемоглобинобразователями или угарным газом, а также при высотной болезни;
- наибольшее количество летальных исходов сосредоточено в странах с морским климатом [24–27];
- наиболее тяжело болезнь протекает при наличии хронических заболеваний дыхательной, сердечно-сосудистой системы и эндокринопатиях [27–30].
Это обосновывает следующую модель патогенеза.
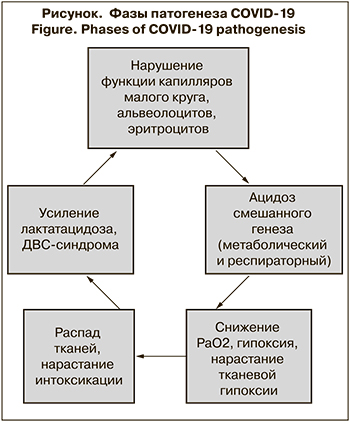
- Проникая в организм через дыхательные пути, коронавирус взаимодействует с рецепторами АПФ-2, которые находятся в капиллярах легких. Фиксация вируса вызывает повреждение стенки, что приводит к агрегации тромбоцитов с последующим закономерным формированием тромба. Нарушение кровотока в капиллярах приводит к дистрофическим изменениям в альвеолоцитах и их гибели при продолжении инфекционного процесса. Этим объясняется наличие изменений по типу «матового стекла» и/или очагов фиброза у некоторых пациентов с бессимптомным или стертым течением инфекции при небольшой активности инфекционно-воспалительного процесса, возможно, связанного с низкой дозой вируса или особенностями иммунного ответа. Распад тканей стимулирует повышение активности лейкоцитов и провоспалительных цитокинов, активность которых направлена на лизис поврежденных и инфицированных клеток.
- В кровотоке коронавирус взаимодействует с рецепторами эритроцитов CD147, что приводит к нарушению основной их функции – передачи кислорода тканям. Такие же нарушения характерны и для средиземноморских гемоглобинопатий, что может объяснять более тяжелое течение болезни у пациентов с наследственно обусловленными анемиями и наибольшее количество летальных исходов в Средиземноморском регионе [31–33].
- Последующий распад эритроцитов сопровождается высвобождением гемоглобина, не способного передавать кислород тканям. Гипоксия приводит к смещению кислотно-основного равновесия крови в сторону ацидоза, что ухудшает состояние органов и тканей, в первую очередь тех, в которых уже имеются изменения.
- Разрушение клеток, участвующих в снабжении тканей кислородом, и гипоксия приводят к накоплению продуктов распада, являющихся эндогенными токсинами.
- Гипоксемия приводит к нарастанию лактатацидоза, что вызывет усиление ДВС-синдрома и выраженно ухудшает состояние больных с сердечно-сосудистой патологией и сахарным диабетом 2-го типа.
- Взаимодействие вируса с рецепторами АПФ-2 в других органах (почках, печени, головном мозге, пищеводе, подвздошной кишке) приводит к нарушениям кровоснабжения по аналогичному механизму. Степень поражения тканей зависит от числа рецепторов АПФ-2 в них и имеющихся предшествующих изменений.
 На сегодняшний день этиотропного лечения COVID-19 c доказанной клинической эффективностью не существует. Между тем патогенетический подход в лечении коронавирусной инфекции мог бы существенно облегчить ситуацию. Первые заключения о преобладании гемической гипоксии у больных COVID-19 сделали китайские врачи, затем их американские коллеги, однако эти данные не подтверждены клинически и являются лишь гипотезами, сформированными при помощи компьютерного моделирования. Однако именно такой подход объясняет неэффективность проводимой ИВЛ-терапии, увеличение смертности при превышении доз гидроксихлорохина и необходимость использования аппаратов экстракорпоральной мембранной оксигенации (ЭКМО) [31–35].
На сегодняшний день этиотропного лечения COVID-19 c доказанной клинической эффективностью не существует. Между тем патогенетический подход в лечении коронавирусной инфекции мог бы существенно облегчить ситуацию. Первые заключения о преобладании гемической гипоксии у больных COVID-19 сделали китайские врачи, затем их американские коллеги, однако эти данные не подтверждены клинически и являются лишь гипотезами, сформированными при помощи компьютерного моделирования. Однако именно такой подход объясняет неэффективность проводимой ИВЛ-терапии, увеличение смертности при превышении доз гидроксихлорохина и необходимость использования аппаратов экстракорпоральной мембранной оксигенации (ЭКМО) [31–35].
Приводим клинические примеры (все пациенты находились на амбулаторном лечении), демонстрирующие особенности течения COVID-19, отличающие его от других ОРИ.
Клинический пример 1.
Пациентка Н., 43 лет. Диагноз: U07.1. Коронавирусная инфекция CОVID-19 средней степени тяжести, вирус идентифицирован. Осложнения: двусторонняя полисегментарная пневмония, дыхательная недостаточность 1-й степени. По данным КТ, поражение легочной ткани – 25%. Наличие контакта отрицает, болеет 5-й день.
Клиническая картина: лихорадка 38,5 оС, сухой, надсадный кашель. Сатурация кислорода (SaO2 ) – 94%, ЧДД – 20 в минуту, ЧСС – 110 ударов в минуту, ощущение тяжести в грудной клетке, с 3-го дня болезни – секреторная диарея.
Лечение: азитромицин 250 мг в сутки, левофлоксацин 500 мг в сутки, осельтамивир 75 мг 2 раза в сутки, парацетамол 500–1000 мг при необходимости, обильное теплое питье. С 3-го дня болезни – субфебрильная лихорадка 37,3 °С, SaO2 – 92%, нарастающая одышка. От госпитализации в профильный стационар пациентка категорически отказалась. В связи с нарастанием гипоксемии введен рибофлавин (витамин В2 ) 1% 1 мл внутривенно, однократно. Одышка купирована. Пациентка продолжает лечение. SaO2 – 96%, сохраняется субфебрильная лихорадка 37,3 °С. Добавлены антиоксидантная (аскорбиновая кислота, витамины А, Е и D) и дезинтоксикационная терапия (Зостерин-Ультра 60% 0,5–2 раза в сутки).
Клинический пример 2.
Пациентка Х., 33 года. Диагноз: U07.1. Коронавирусная инфекция COVID-19 средней степени тяжести, вирус идентифицирован. Интоксикационный синдром. Контакт с инфицированным SARS-CoV-2 25.03.2020. Больна с 02.04.2020.
Клиническая картина: в 1-й день болезни лихорадка 38,0 °С; головная боль, локализованная в правой теменно-височной области, с иррадиацией в правый глаз; одышки нет. SaO2 – 98%, ЧДД – 16 в минуту, ЧСС – 98 ударов в минуту.
Лечение: риамиловир по 250 мг 3 раза в день, левофлоксацин 500 мг 1 раз в день, парацетамол 500 мг при необходимости. С 3-го дня болезни – субфебрильная лихорадка 37,5 °С; головная боль усиливается, без светлых промежутков, соответствует мигренозному статусу; присоединяется диарея; кратковременный до 5 мин парез правой руки с последующим полным восстановлением функции. К терапии добавлен энтеросорбент кремния диоксид коллоидный по 1 столовой ложке (растворенной в стакане теплой воды) в сутки. С 5-го дня болезни купированы головная боль, диарея; субфебрильная лихорадка не выше 37,3 °С. С 6-го дня пациентка предъявляет жалобы на отсутствие обоняния, сухой кашель, слабость. Отмечена тахикардия 110 ударов в минуту, одышки нет. Терапия продолжена. Результат анализа периферической крови: эозино- и базофилия – незначительно, по остальным показателям – без отклонений от референсных значений. Улучшение общего самочувствия к 27.04.2020: улучшение сна, повышение активности, тахикардии нет, SaO2 – 98%.
Клинический пример № 3.
Пациент С., 47 лет. Диагноз: U07.1 Коронавирусная инфекция COVID-19 средней степени тяжести, вирус идентифицирован. Интоксикационный синдром. Болен с 24.04.2020.
Клиническая картина: жалобы на озноб, повышение температуры тела днем до 37,4 °С, ночью – до 38,4 °С, интенсивную головную боль, боли в мышцах и суставах. Носовое дыхание свободное, болей в горле нет.
Лечение: промывание носовых ходов изотоническим солевым раствором, физические методы охлаждения, обильное теплое питье. С вечера 25.04.2020 получает Зостерин-Ультра 60% 0,5 на ночь, осельтамивир 75 мг 2 раза в сутки, с 26.04.2020 – левофлоксацин по 500 мг 1 раз в день, комбинацию витаминов А и Е 1 раз в сутки. Спустя двое суток лихорадка купирована, отмечается сухой кашель со скудной мокротой, ЧДД – 16 в минуту, SaO2 – 98%. По данным КТ, инфильтративных изменений в легких нет.
Из представленных клинических наблюдений следует, что при добавлении энтеросорбирующих препаратов практически в начале лечения в 2 случаях удалось купировать острые симптомы интоксикации. В 1 случае добавление объемной антиоксидантной терапии способствовало общему улучшению самочувствия и нормализации показателей сатурации. В 1 из 3 случаев добавление рибофлавина позволило купировать одышку и повысить показатель SaO2 с 94 до 96%. Безусловно, антиоксидантная и сорбирующая терапия не могут стать основой лечения, тем не менее целый ряд свойств препаратов данной группы способен существенно облегчить течение и прогноз заболевания (табл. 1).
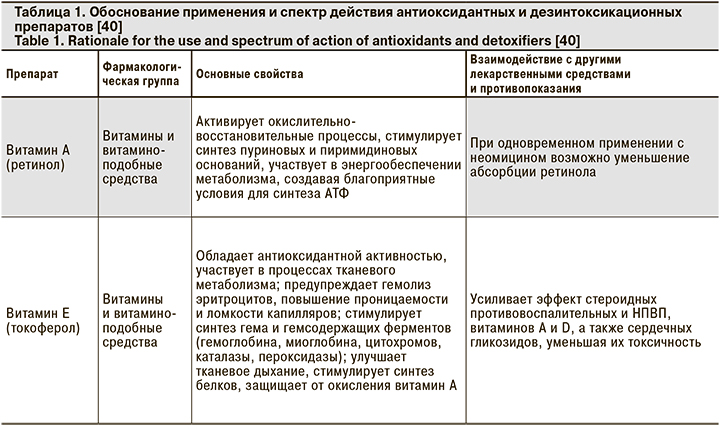
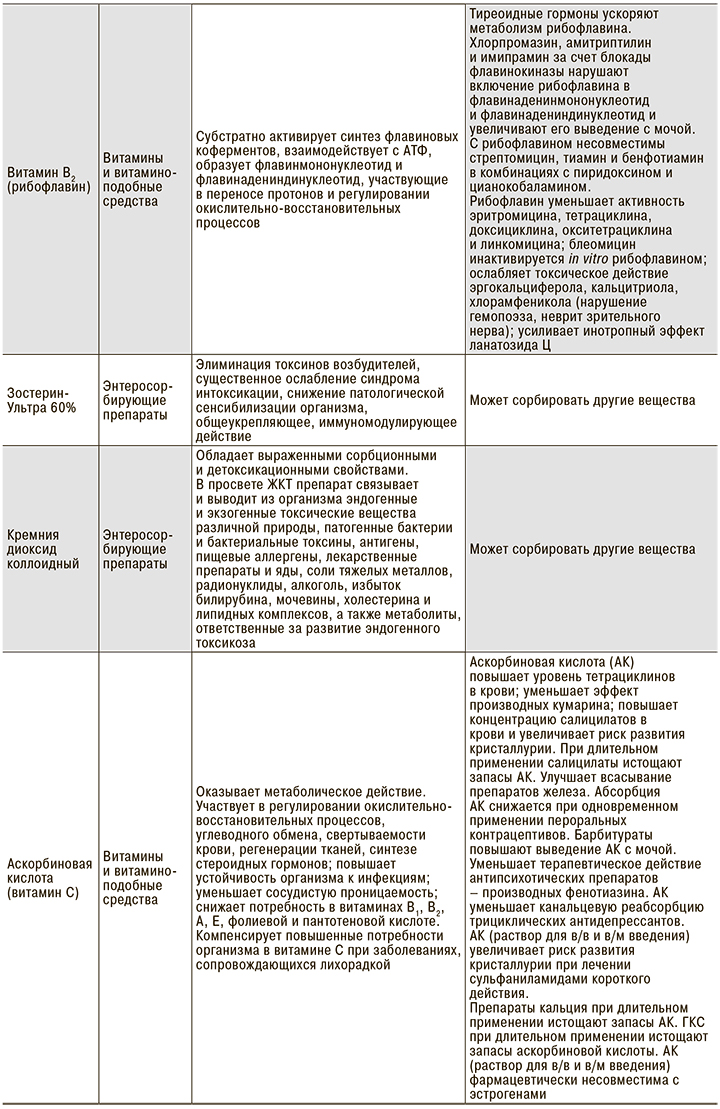
В этой связи патогенетическое лечение пациентов с COVID-19 выглядит иначе, чем стандартно принятые подходы к терапии ОРИ. Основными необходимыми пунктами представляются следующие [36–39]:
- Профилактика и лечение гипоксемии: аскорбиновая кислота (5% раствор 5–10 мл в/в), рибофлавин, глутатион, гипербарическая оксигенотерапия.
- Дезинтоксикационная терапия: Зостерин-Ультра, диоксид кремния коллоидный или другие энтеросорбенты; гемосорбция, плазмаферез.
- Улучшение кровотока: прямые антикоагулянты.
- Профилактика и лечение гемолитической анемии, подавление гиперстимуляции иммунных реакций: препараты на основе хлорохина.
- Профилактика активации вторичной флоры – антибиотикотерапия.
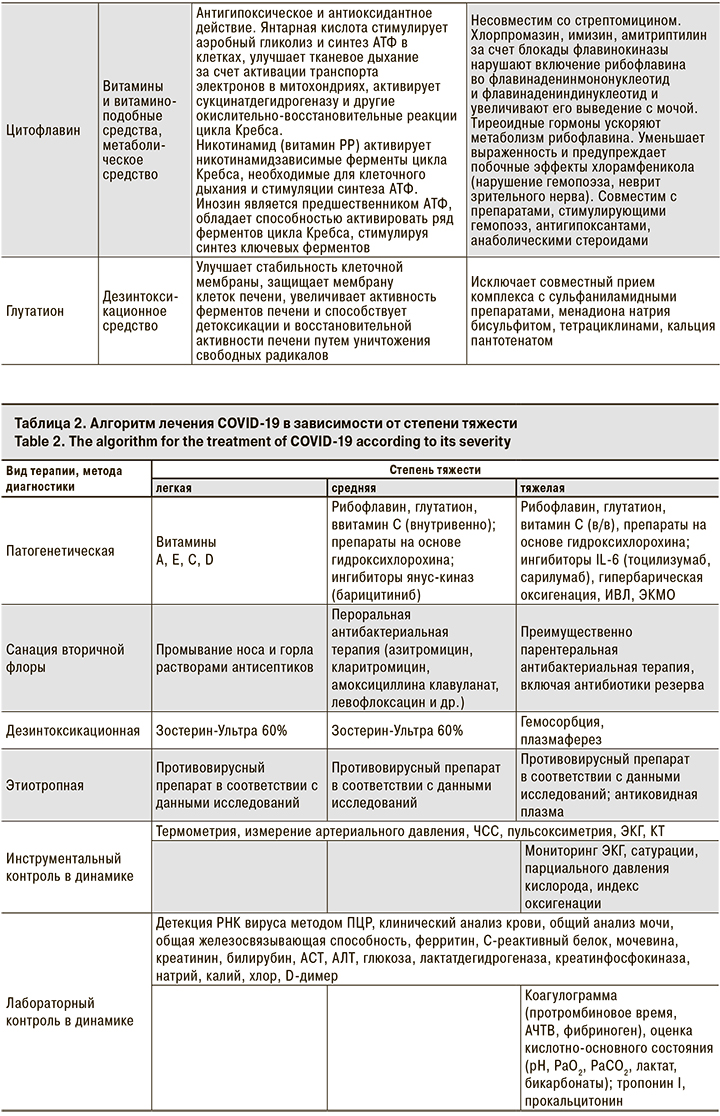
Алгоритм, включающий дезинтоксикационную и антиоксидантную терапию, представлен в табл. 2.
Учитывая результаты наблюдений, при тяжелом и среднетяжелом течении заболевания лечение следует начинать еще на догоспитальном этапе, при транспортировке пациента. Показаны ингаляции кислородом и антиоксидантная терапия. У тяжелых пациентов возможно применение закиси азота. Этот вопрос в настоящее время обсуждается специалистами.
Заключение
Сложившаяся в мире эпидемическая ситуация диктует необходимость поиска новых схем лечения практически ежедневно. На наш взгляд, основная задача в текущий период – предотвратить переход течения заболевания у пациентов с COVID-19 от легкой формы к тяжелой и крайне тяжелой, а также выработать эффективные меры профилактики отдаленных последствий для бессимптомных носителей.
Антиоксидантная, дезинтоксикационная и энтеросорбирующая терапия эффективна в снижении выраженности и даже купировании интоксикационного и гипоксического синдромов, оказывает благоприятное влияние на состояние пациентов с COVID-19. Безусловно, выбор препаратов для патогенетической терапии нужно проводить индивидуально, с учетом физикальных, лабораторных и инструментальных показателей, а также сопутствующей патологии и проводимой базисной терапии.


