Крымская геморрагическая лихорадка (КГЛ) эндемична для южных областей Казахстана (Туркестанской, Жамбылской и Кызылординской), где в общей сложности ежегодно регистрируется от 5 до 15 случаев этого заболевания. В среднем летальность составляет 13,8% [1].
Рибавирин является рекомендованным ВОЗ препаратом для этиотропного лечения КГЛ1 [2–3]. Тем не менее, рандомизированные клинические исследования по применению рибавирина в соответствии с протоколом GCP не проводились. Существуют только публикации по его применению в обсервационных исследованиях2, данные которых противоречивы [4–7]. Вместе с тем ряд исследователей отмечает его эффективность при применении на ранних стадиях болезни (в предгеморрагический период), а также при сравнении с историческим контролем (до начала его применения в терапии КГЛ) [8, 9]. В Казахстане его назначают согласно схеме, рекомендованной ВОЗ [10].
Особенностью стационарной помощи больным КГЛ в Республике Казахстан является комбинированнное применение рибавирина и свежезамороженной плазмы (СЗП) реконвалесцентов [8, 10]. Рекомендуется введение 100–300 мл (1–2 лечебные дозы) в максимально ранние сроки. Применяется СЗП с титром антител к вирусу КГЛ не менее 1:400. При введении иммунной плазмы реконвалесцентов от донора в организм больного поступают как антитела к вирусу, так и факторы свертывания крови. Критериями эффективности лечения являются регрессия клинических проявлений, нормальные значения лабораторных показателей и температуры. Данная схема оказывает положительный терапевтический эффект и способствует улучшению прогноза заболевания [10]. Так, при использовании для терапии КГЛ рибавирина и СЗП доноров и (в некоторых случаях) комбинации его и СЗП реконвалесцентов летальность от КГЛ, составлявшая в 2009 г. 36,3%, к 2018 г. снизилась в 4–5 раз [1]. В 2014 г. плазма реконвалесцентов включена в Национальный протокол диагностики и лечения КГЛ Республики Казахстан [8].
В настоящее время для оценки эффективности терапии все чаще используют клинико-экономический анализ. Особенно актуально это при выборе разных методов, имеющих сопоставимую клиническую эффективность или диагностическую информативность [11, 12]. В последнее время этот метод анализа стал также применяться для оценки эффективности терапии ряда инфекционных и паразитарных заболеваний3,4 [13]. При КГЛ такие исследования ранее не проводились. Между тем появление новой схемы ее лечения обусловило необходимость выполнения подобного исследования.
Цель исследования – фармакоэкономический анализ эффективности терапии КГЛ с использованием рибавирина и плазмы реконвалесцентов.
Материалы и методы
В исследование были включены 94 больных КГЛ, проходивших стационарное лечение в Городской инфекционной больнице г. Шымкента, районных больницах эндемичных областей юга Казахстана (Туркестанской, Жамбылской и Кызылординской), в 2001–2019 гг.
Больные были разделены на 3 группы, сопоставимые по полу, возрасту, срокам поступления в стационар от момента начала заболевания и тяжести заболевания.
32 пациента вошли в 1-ю группу (группа сравнения). В их лечении использовали только базисную патогенетическую терапию, применяемую при лечении КГЛ согласно Клиническому протоколу диагностики и лечения КГЛ, используемому в Республике Казахстан [10]. Пациентам 2-й группы (n = 32) назначали рибавирин в сочетании с базисной патогенетической терапией. Пациенты 3-й группы (n = 30) получали комбинированную терапию, включающую рибавирин и иммунизированную плазму, в сочетании со стандартной терапией (табл. 1).
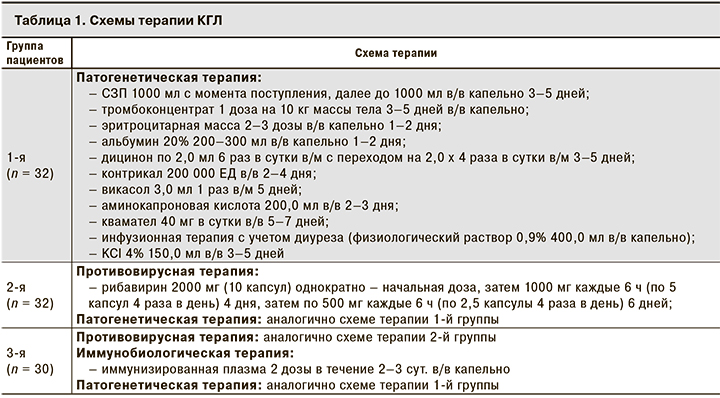
Статистическую обработку полученных результатов проводили с использованием статистической программы SPSS Statistica, версия 20. Использовали методы описательной статистики, критерий Стьюдента (включая парный критерий Стьюдента). Полученные различия считали статистически значимыми при p < 0,05.
Для экономической оценки эффективности терапии был использован анализ общей (полной) стоимости болезни – COI (costofillnesses), определяемый по совокупности прямых (directcosts – DC) и непрямых (indirectcosts – IC) затрат.
Для расчета полной стоимости КГЛ использовали формулу:
COI = DC + IC
Расчет полной стоимости КГЛ в каждой группе выполняли по формуле:
COI = (COI1 + COI2 + COI3 +…+ COIn)/n,
где COI1,2,3…n – показатель стоимости КГЛ каждого больного КГЛ;
n – число пациентов в группе [14].
При определении прямых затрат учитывали расходы на пребывание пациента в стационаре по стоимости 1 койко-дня в городской больнице г. Шымкент, актуальной на 01.06.2019. Для этого из стоимости 1 койко-дня пребывания в инфекционном боксированном отделении (10 356 тенге) вычитали заложенную в эту сумму стоимость медикаментов (3534 тенге), умножали полученный результат (6822 тенге) на длительность стационарного лечения и суммировали с реальными затратами на лекарственные средства во время стационарного лечения в каждой группе. Под непрямыми затратами (IC) подразумевались расходы на оплату листов временной утраты трудоспособности работающим пациентам, основанные на средней заработной плате в г. Шымкент на 01.06.2019, равной 40 232,9 тенге. Для определения IC в каждой группе использовали формулу:
IC = (IC1 + IC2 + IC3 +…+ ICn)/n,
где IC1,2,3…п – непрямые затраты на каждого пациента;
n – число пациентов в группе [14].
Принимая во внимание то, что анализу подвергали разные схемы, имеющие одни и те же конечные цели: добиться наиболее скорого выздоровления и не допустить развития осложнений, анализ по критерию СЕА (cost-effectiveness analysis) позволил оценить как затраты на лечение, так и его эффективность [15].
При клинической оценке результатов терапии КГЛ в качестве параметра эффективности была взята длительность стационарного лечения у выздоровевших пациентов.
Для каждой альтернативной схемы лечения КГЛ при проведении этого анализа рассчитывали соотношение «затраты–эффективность» по формуле:
CEA = (DC + IC)/Ef,
где CEA – соотношение «затраты–эффективность» (расходы на единицу эффективности);
Ef – эффективность лечения [14].
В нашем исследовании за эффективность была принята относительная величина, обратная сумме абсолютных чисел средней длительности пребывания в стационаре (дни) и частоты летальных исходов (%) в каждой группе [14, 16]. Наиболее эффективным является терапия с наименьшим показателем CEA.
Результаты
Анализ динамики клиническо-лабораторной картины заболевания показал, что по большинству параметров (длительность лихорадки, продолжительность периода тромбоцитопении менее 100 х 109/л и геморрагического периода, длительность пребывания пациентов в стационаре) существенными преимуществами перед патогенетической терапией (1-я группа), обладала схема терапии, включающая дополнительно рибавирин и иммунизированную плазму (3-я группа). Она также обладала преимуществами по сравнению со схемой, включавшей дополнительно только рибавирин (2-я группа), по длительности геморрагического периода, продолжительности периода тромбоцитопении менее 100 х 109/л и стационарного лечения. Длительность стационарного лечения в 1-й группе оказалась наибольшей (13,8 ± 0,45 койко-дня), а в 3-й – минимальной (9,8 ± 0,34 койко-дня). Различия между группами оказались статистически значимыми (табл. 2). Несмотря на отчетливую тенденцию сокращения числа летальных исходов при использовании рибавирина и рибавирина совместно с иммунизированной плазмой, статистически достоверной разницы по этому параметру между группами не обнаружено.

Для оценки затрат на лекарственные препараты была определена средняя стоимость медикаментов, затраченных на каждого пациента в каждой группе. Выяснилась, что, более высокие затраты на 1 больного в 3-й группе по сравнению с 1-й и 2-й не привели к статистически значимой разнице в расходах на лекарственные препараты, DC, а также COI. Достоверные различия были получены при изучении только IC, связанных с сокращением длительности периода нетрудоспособности во 2-й и 3-й группах по сравнению с 1-й (табл. 3).
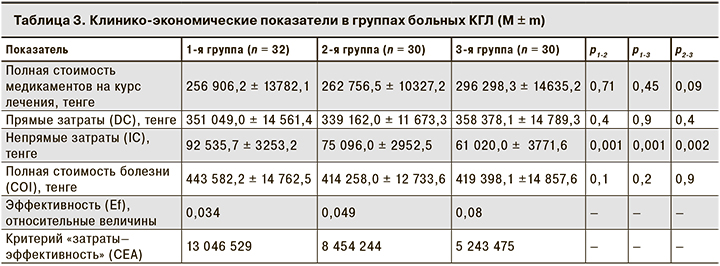
Клинико-экономический анализ по критерию CEA показал, что наименьшие затраты на единицу эффективности приходились на пациентов 3-й группы: они были в 2,5 раза ниже, чем в 1-й группе, и в 1,5 раза ниже, чем во 2-й. Затраты на единицу эффективности во 2-й группе оказались в 1,6 раза ниже, чем в 1-й.
Таким образом, можно констатировать не только клиническую, но и определенную экономическую целесообразность использования комбинации рибавирина и иммунизированной плазмы реконвалесцентов КГЛ в комплексной терапии этого заболевания, основу которой составляет патогенетическая терапия. Это выражается в сокращении периода клинико-лабораторных проявлений заболевания, длительности пребывания в стационаре, отсутствии существенных различий в стоимости лекарственных препаратов, затраченных на лечение, достоверном сокращении IC на терапию и снижением показателя COI.
Обсуждение
Несмотря на продемонстрированную клиническую и экономическую целесообразность комбинированной терапии больных КГЛ, в оценке эффективности этих схем остается еще много вопросов. Это связано со сложностью проведения рандомизированных исследований, подразумевающих оценку сравнительной эффективности стандартной патогенетической терапии и схем лечения, дополненных этиотропной и/или специфической иммунной терапией, даже не имеющей основательной доказательной базы в пользу ее эффективности [5, 6]. Эти доводы основываются на принципах качественной клинической практики, медицинской этики и гуманизма, а также учитывают высокую летальность при КГЛ [15]. Тем не менее целый ряд клинико-лабораторных показателей, а также результаты клинико-экономического анализа свидетельствуют в пользу целесообразности применения рибавирина и иммунизированной плазмы в лечении пациентов с КГЛ на современном этапе развития инфектологии. Весьма перспективные результаты в экспериментальных моделях КГЛ на лабораторных животных были получены при использовании фавипинавира и моноклональных антител [17]. Поэтому мы надеемся, что в недалеком будущем появится возможность провести сравнительные рандомизированные клинические исследования для оценки эффективности различных схем этиотропной и специфической терапии этого заболевания.
Выводы
- Применение рибавирина и иммунизированной плазмы в лечении КГЛ способствует более быстрому нивелированию клинико-лабораторных проявлений заболевания и снижает риск развития летального исхода.
- Схема терапии КГЛ, включающая рибавирин и иммунизированную плазму, сокращает прямые и непрямые затраты на лечение, а также затраты, приходящиеся на единицу эффективности (CEA), и является экономически выгодной.


