Распространение антибиотикорезистентности (АР) является одной из самых острых проблем в мире. Она несет в себе не только серьезные экономические, но и биологические угрозы. По данным международных экспертов, за год от инфекций, вызванных антибиотикрезистентными микроорганизмами, умирает более 700 000 человек, из них 22 000 смертей приходятся на страны Европы. К 2050 г. эта цифра может увеличиться до 10 млн человек [1–3].
При оказании медицинской помощи в условиях стационара ситуация усугубляется ускоренной селекцией полирезистентных микроорганизмов, устойчивых к широкому спектру антимикробных препаратов (АМП), что является результатом их неограниченного применения и проявлением побочного экологического эффекта – так называемая концепция «параллельного ущерба» [4, 5]. В зоне особого внимания находятся такие классы АМП, как цефалоспорины (ЦС) III поколения [6, 7], фторхинолоны [8, 9] и карбапенемы [10].
По данным многоцентрового эпидемиологического исследования МАРАФОН, проведенного в России в 2013–2014 гг. [11], устойчивость госпитальных штаммов Enterobacteriaceae к ЦС III и IV поколения составила более 70%. Pseudomonas aeruginosa более чем в 50% случаев была устойчива к цефтазидиму, цефепиму, пиперациллину/тазобактаму, меропенему и имипенему, амикацину, большинство штаммов нечувствительно к ципрофлоксацину [12]. Практически все штаммы Acinetobacter baumaniae устойчивы к ципрофлоксацину и ЦС III и IV поколения, до 74,5% штаммов резистентны к карбапенемам. Фенотипом множественной резистентности (MDR) обладали 98% изолятов A. baumaniae, а у 64,4% определен фенотип экстремальной резистентности (XDR) [13]. Нарастает доля MRSA и стафилококков со сниженной чувствительностью к ванкомицину (VRSA/VISA) [5, 14–16], ванкомицинрезистентных энтерококков (VRE) [17], и уже есть сообщения о линезолидрезистентных энтерококках [18, 19].
Данные особенности микроорганизмов существенно ограничивают выбор антибактериальных препаратов для лечения инфекций, вызванных этими штаммами, снижают качество оказания медицинской помощи, значительно повышают стоимость лечения и нередко ведут к летальному исходу.
В сентябре 2017 г. Правительство Российской Федерации утвердило Стратегию предупреждения распространения антибиотикорезистентности в Российской Федерации1. Документ определяет государственную политику по предупреждению и ограничению распространения устойчивости микроорганизмов к АМП, химическим и биологическим средствам. Основные направления реализации поставленных задач представлены на схеме 1 (см. на вклейке).
Основной причиной роста АР и параллельного ущерба от применения АМП является практика бесконтрольного и нерационального их использования (неадекватный выбор показаний к антимикробной терапии, доз, интервалов введения, продолжительности курса). Большая доля АМП в хирургических стационарах расходуется при проведении периоперационной антибиоткопрофилактики (ПАП).
Программы по надзору за использованием АМП являются основным способом сдерживания резистентности, минимизации параллельного ущерба и экономии денежных средств, затрачиваемых на здравоохранение [5, 20].
Цель исследования – снизить потенциальный риск роста АР и повысить качество оказания медицинской помощи путем рационализации применения АМП в стационарах хирургии головы и шеи.
Задача 1 – изменить у врачей стереотипы проведения ПАП.
Задача 2 – повысить качество оказания медицинской помощи пациентам с остро возникшими гнойно-септическими осложнениями (сепсис, менингит, пневмония, пиелонефрит и др.) в стационарах хирургии головы и шеи.
Материалы и методы
Исследование было проведено в рамках мероприятий по сдерживанию развития АР и включало 2 этапа.
1-й этап (ретроспективный) проводили в отделениях хирургического профиля: челюстно-лицевой и пластической хирургии; заболеваний носа и глотки; заболеваний уха (взрослое и детское); онкологии опухолей головы и шеи; заболеваний гортани; детской ЛОР-патологии. Изучали рациональность применения антибактериальных препаратов у больных хирургического профиля в 2016 г. Методом случайной выборки было проанализировано 765 историй болезни.
2-й этап – проспективное исследование. Оценивали изменение потребления АМП в ФГБУ «Научно-клинический центр оториноларингологии» (ФГБУ НКЦО) ФМБА России под влиянием стратегии проспективного аудита с интервенционой составляющей и «обратной связью».
 Результаты
Результаты
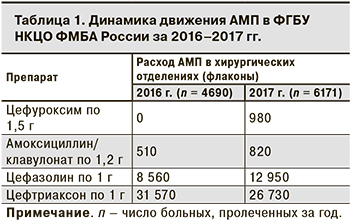
Было установлено, что из АМП чаще всего назначают ЦС III поколения, в частности, цефтриаксон. Помимо лечения гнойно-воспалительных заболеваний его неоправданно широко применяли для ПАП. В то же время с целью ПАП врачи редко назначали защищенные аминопенициллины, ЦС I поколения и совсем не использовали ЦС II поколения (табл. 1). Как правило, 1-е введение АМП при проведении ПАП было в послеоперационном периоде, а ее продолжительность в среднем составляла 5–7 дней. По данным за 2017 г., на фоне внедрения стратегии проспективного аудита с интервенционной составляющей и «обратной связью» отмечена положительная динамика движения АМП по сравнению с 2016 г.
План организационных мероприятий по рациональному использованию АМП для профилактики инфекций в области хирургического вмешательства (ИОХВ) представлен на схеме 2 (см. на вклейке).
В результате работы на протяжении 10 мес. снизилось потребление ЦС III поколения (цефтриаксон) увеличилось включение в схемы ПАП ЦС I–II поколения, защищенных аминопенициллинов (см. табл. 1). Данные за 2017 г. отражают положительную динамику по сравнению с 2016 г.: потребление ЦС I поколения увеличилось на 51%, амоксициллина/клавулоната – на 60%, но общее снижение потребления цефтриаксона за 10 мес. составило только 15,3%.
Такие осложнения, как сепсис, менингит, пневмония, пиелонефрит в ФГБУ НКЦО ФМБА России встречаются нечасто. Если они и развиваются, то, как правило, в послеоперационном периоде. Пациенты, согласно стратификации по риску наличия у них резистентных возбудителей и инвазивного кандидоза [26], относились преимущественно к типу IIIb (госпитализация > 7 дней и/или предшествующее применение АМП). Для них характерен высокий риск выделения БЛРС-продуцентов, неферментирующих грамотрицательных бактерий (Ps. aeruginosa, A. baumaniae и др.), карбапенемрезистентных штаммов и MRSA.
В Центр также нередко поступают больные с хроническими гнойными средними отитами с отореей, у которых, помимо S. aureus и Enterobacteriaceae, очень высок риск выделения Ps. аeruginosaе2, что требует безотлагательного назначения препаратов с антисинегнойной активностью. По данным микробиологического мониторинга, синегнойная палочка у этих больных обладала хорошей чувствительностью ко всем антисинегнойным препаратам (цефтазидим, цефипим, амикацин, гентамицин, меропенем, имипенем), но встречались штаммы, устойчивые к ципрофлоксацину.
Учитывая это, был создан запас АМП, включающий карбапенем с антисинегнойной активностью, применяющийся также для лечения инфекций ЦНС (меропенем), гликопептиды (ванкомицин) и/или оксозолидиноны (линезолид), антисинегнойные ЦС (цефепим и цефтазидим). Неснижаемое количество каждого АМП было рассчитано на лечение 1 пациента в течение не менее 3 сут. Чтобы обеспечить готовность взять пробы крови для определения возбудителя бактериологическим методом с определением чувствительности к антибиотикам и другим лекарственным препаратам, в наличии всегда должны быть транспортные питательные среды для культивирования анаэробных и аэробных микроорганизмов (не менее 2 флаконов каждого типа сред).
Одновременно был издан внутренний приказ «О номенклатуре и объеме запаса антибактериальных препаратов и сред на стерильность», где четко прописаны место хранения; лица, ответственные за хранение и пополнение запасов при их расходе; сроки замены для питательных сред и АМП.
Обсуждение
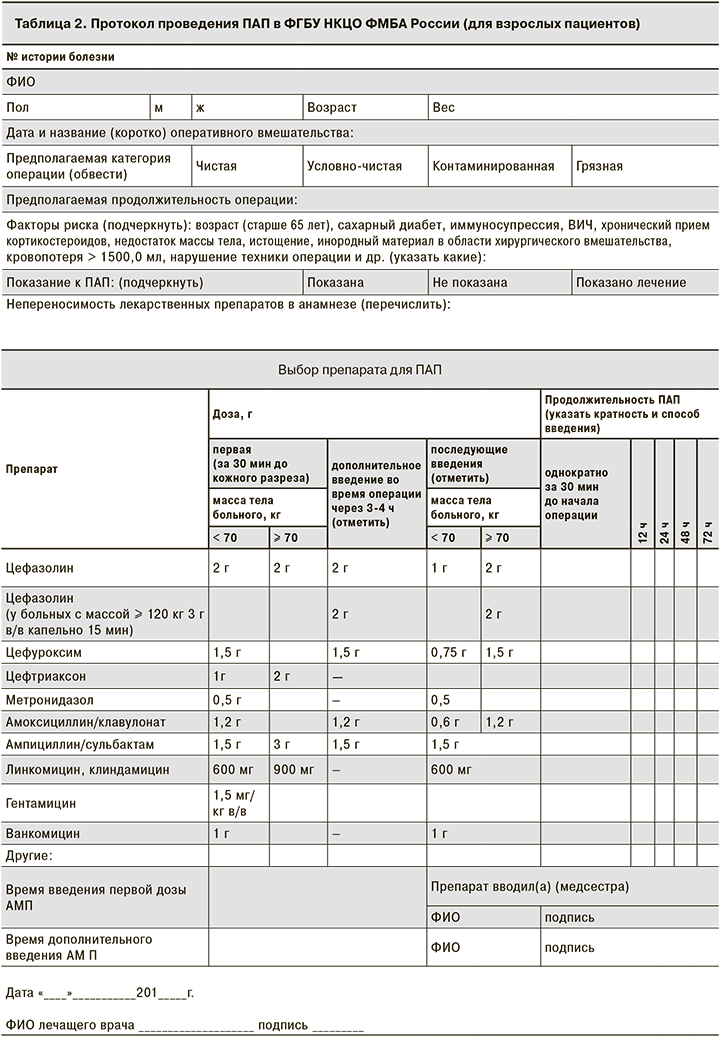
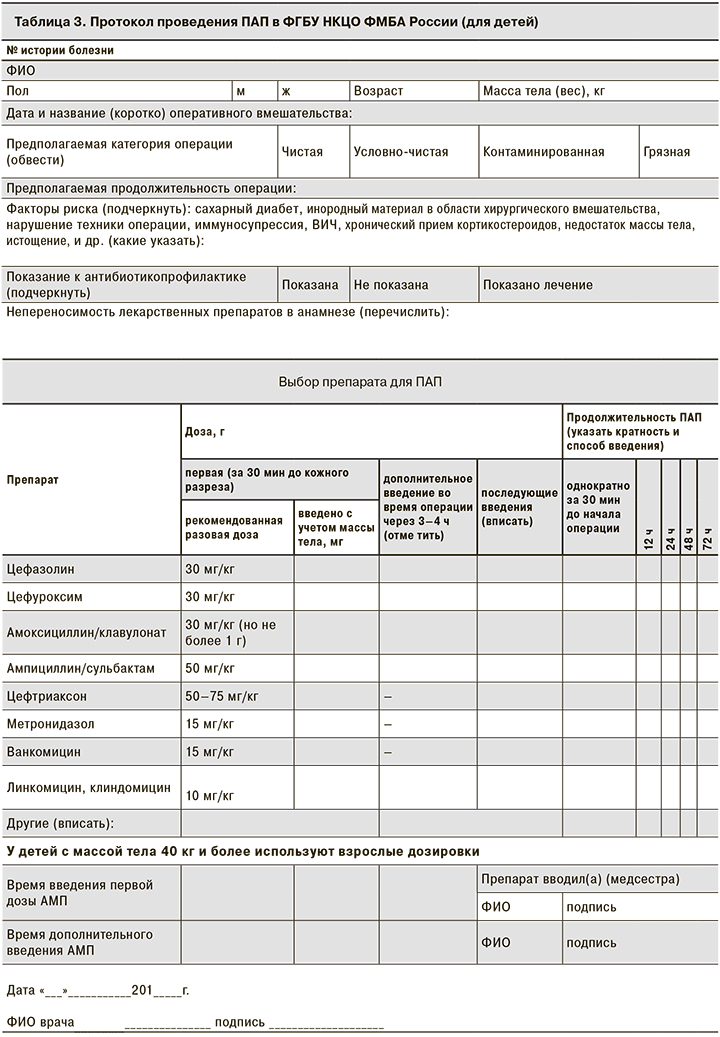
При создании протоколов ПАП были учтены наиболее значимые моменты, от которых зависит эффективность ПАП: тип операции, масса тела пациента, факторы риска развития ИОХВ; выбор дозы в зависимости от массы тела, указания для дополнительного интраоперационного введения АМП, продолжительность ее проведения. Особого внимания заслуживают графы с указанием времени введения первой дозы АМП относительно начала оперативного вмешательства и повторного интраоперационного введения. Если в последнем случае данные о своевременности ведения АМП можно найти в анестезиологической карте, где фиксируются все лекарственные препараты, введенные во время операции, то данные о введении первой дозы АМП, как правило, не фиксируются в истории болезни, что затрудняет оценку качества оказания медицинской помощи: соблюдается ли основной принцип проведения ПАП – введение АМП за 30 мин до начала операции – и связано ли ИОХВ, (если такое возникло) с несвоевременностью введения АМП или имеются другие причины.
Неотъемлемой частью внедрения ПАП был регулярный аудит историй болезни. В случае обнаружении ошибок при заполнении протоколов ПАП и/или выбранных схем лечения/профилактики проводился разбор конкретных клинических ситуаций с лечащими врачами и заведующими отделениями.
Вопрос о необходимости проведения организационных мероприятий по формированию банка АМП «резерва» и транспортных сред был связан с отсутствием готовности оказать экстренную медицинскую помощь больным с остро возникшими гнойно-септическими состояниями. Подобная ситуация достаточно распространена как в обычных городских больницах, так и в крупных хирургических центрах.
Как известно, антибиотикотерапию (АБТ) таким больным необходимо назначать неотложно сразу после диагностики инфекции, до получения результатов бактериологического исследования. При тяжелом сепсисе адекватный АМП должен быть введен в течение первого часа после установления диагноза, сразу после взятия материала для микробиологического исследования. АБТ должна наиболее полно охватывать всех потенциальных возбудителей, характерных для той или иной локализации инфекционного процесса, и учитывать возможную АР [26, 27].
Если подобная ситуация складывается в дневные часы, проблем с проведением обследования и началом АБТ обычно не возникает. Но при нештатной ситуации (в вечернее и ночное время или в выходные дни), возникали затруднения, так как АМП «резерва» и транспортные питательные среды отсутствовали в отделениях, что требовало экстренного вызова сотрудника аптеки и лаборатории. При этом увеличивалось время от момента постановки диагноза до начала адекватной АБТ.
При создании в стационарах неснижаемых запасов резервных АМП и транспортных питательных сред предпочтенье следует отдавать коммерческим средам [26]. В нашем случае использовались флаконы BacT/ALERT.
Опыт показывает, что наиболее удобное место хранения АМП «резерва» и питательных сред – отделение реанимации и интенсивной терапии. Но использоваться они должны не только у пациентов этого отделения, но при необходимости передаваться по дежурству в другие отделения для больных, которые по тяжести состояния не нуждаются в переводе в ОРИТ, но требуют назначения/смены АБТ в экстренном порядке.
При определении количества АМП «резерва» учитывали частоту развития гнойно-воспалительных осложнений в стационаре, а номенклатура АМП «резерва» основывалась на данных о локализации инфекции, выделении вероятного возбудителя, риске инфицирования полирезистентными возбудителями и результатах локального микробиологического мониторинга.
Выводы
- Внедрение стратегии проспективного аудита с интервенционой составляющей и «обратной связью» позволяет значительно изменить стереотипы проведения ПАП, снизить назначение не по показаниям ЦС III поколения как одной из самых неблагонадежных групп АМП с точки зрения концепции «параллельного ущерба».
- Процесс внедрения ПАП очень трудоемок. Врачи с трудом отказываются от стереотипов, для преодоления которых необходима постоянная «обратная связь» между хирургами и клиническим фармакологом и/или комитетом по надзору за использованием АМП (что предпочтительней), куда должны входить клинический фармаколог, специализирующийся на инфекционных болезнях, эпидемиолог, клинический микробиолог и, возможно, специалист по инфекционному контролю.
- Внедрение протоколов ПАП в хирургических стационарах, в том числе стационарах хирургии головы и шеи, позволяет врачам рационально назначать АМП больным, подвергшимся хирургическому вмешательству, а также контролировать обоснованность и правильность выбора тактики назначения АМП.
- Наличие запаса АМП «резерва» и сред обеспечивает своевременность забора крови для бактериологического исследования и назначения адекватной АБТ в течение первого часа от момента постановки диагноза в любой период времени, что обеспечивает высокое качество медицинской помощи больным с сепсисом (септическим шоком), менингитом, нозокомиальной пневмонией и др.
- Организация в стационаре запаса антибиотиков «резерва» и транспортных сред позволяет использовать деэскалационный подход при лечении тяжелых инфекций, повышает качество оказания медицинской помощи и может быть рекомендована не только для стационаров хирургии головы и шеи, но и для многопрофильных хирургических стационаров. При этом номенклатура АМП «резерва» должна быть шире.


