Эндогенными антимикробными пептидами (АМП) называют класс молекул иммунной системы, действие которых позволяет предотвратить микробную инвазию [1]. На сегодняшний день известно более 500 эндогенных АМП, участвующих в защите эпителиальных тканей животных и человека [2]. Несмотря на то что АМП являются древнейшим фактором врожденного иммунитета и обнаруживается повсеместно в организме человека, эти соединения начали изучать всего несколько десятилетий назад. АМП называют натуральными антибиотиками, так как они оказывают антимикробное действие на широкий спектр микроорганизмов – вирусы, бактерии, грибы и простейшие. Большинство этих пептидов относятся к катионным белкам, разнообразным по аминокислотной последовательности, вторичной структуре и механизму действия на клетки микроорганизмов, результатом которого является деструкция цитоплазматической мембраны. В настоящее время АМП рассматривают в качестве альтернативы известным антибиотическим и иммуномодулирующим препаратам.
Заболевания, возникающие при попадании патогенов в респираторный тракт, объединены в большую группу респираторных инфекционно-воспалительных заболеваний (РИВЗ). Ротоглотка является одним из первых барьеров на пути патогенных микроорганизмов и в здоровом состоянии заселена многими видами резидентных микробов. Наше предыдущее исследование было посвящено изучению видового состава и антибиотикочувствительности микробиоты, населяющей ротоглотку при РИВЗ: выявлены наиболее часто встречающиеся виды/роды микроорганизмов – Staphylococcus aureus, Enterococcus spp., Streptococcus agalactiae, Neisseria spp. и Streptococcus pyogenes [3]. Одним из важных факторов антимикробной защиты ротоглотки является слюна [4]. В здоровом состоянии у взрослого человека выделятся порядка 0,3–0,4 мл слюны в минуту или 2–2,5 л/сут. Механизмы защиты ротоглотки не всегда срабатывают, что может быть обусловлено как факторами окружающей среды, так и состоянием иммунитета. В слюне обнаружено порядка 45 идентифицируемых антимикробных генных продукта слизистой оболочки, слюнных желез и нейтрофилов. Особо можно отметить крупные семейства антимикробных пептидов, такие как дефензины (α- и β-) и гистатины [5, 6]. В табл. 1 представлены данные, касающиеся спектра АМП, обнаруженных в слюне человека, а также их свойств (молекулярных масс, химических особенностей, концентраций) [7–17] и изменения уровня экспрессии при РИВЗ в соответствующих локусах [18–33]. Видно, что концентрации АМП в слюне варьируют в широком диапазоне от 10-2 (адреномедуллин) до 105 (гистатины) нг/мл. К настоящему времени накоплен значительный клинический материал о взаимосвязи между синтезом АМП и многими заболеваниями, в том числе РИВЗ. Из данных табл. 1 следует, что нет общей закономерности в изменении уровней АМП при РИВЗ, они могут повышаться или понижаться в зависимости от вида АМП и нозологической формы заболевания. Очевидно также, что в слюне, как и в прочих секретах и локусах, действует весь спектр АМП одновременно, поэтому логичным подходом к определению роли АМП при инфекционно-воспалительных заболеваниях является оценка их совокупного действия на референс-культуры микроорганизмов [34, 35]. В этой связи целью настоящего исследования явилось изучение совокупной активности АМП фракции слюны во взаимосвязи с соответствующей локусу микрофлорой и клинической картиной, что даст возможность оценить роль АМП в иммунной защите ротоглотки при РИВЗ.

Материалы и методы
 В исследование включены 203 пациента в возрасте от 2 до 64 лет с РИВЗ: хронический ринит/хронический ринофарингит (ХРФ), аллергический ринит (АР), острый назофарингит (ОНФ), болезнь аденоидов/миндалин (БАМ), хроническая обструктивная болезнь легких (ХОБЛ), бронхиальная астма (БА), ангина/острый тонзиллит (АТ) с различной степенью тяжести, соответствующей разработанной нами шкале (табл. 2).
В исследование включены 203 пациента в возрасте от 2 до 64 лет с РИВЗ: хронический ринит/хронический ринофарингит (ХРФ), аллергический ринит (АР), острый назофарингит (ОНФ), болезнь аденоидов/миндалин (БАМ), хроническая обструктивная болезнь легких (ХОБЛ), бронхиальная астма (БА), ангина/острый тонзиллит (АТ) с различной степенью тяжести, соответствующей разработанной нами шкале (табл. 2).
Контрольную группу составили 29 добровольцев без симптомов РИВЗ в возрасте от 5 до 70 лет.
Для микробиологических исследований мазков из ротоглотки группы обследованных делили следующим образом: подгруппа 1 – дети (контроль; п = 11), подгруппа 2 – дети с РИВЗ (n = 143), подгруппа 3 – взрослые (контроль; n = 18) и подгруппа 4 – взрослые с РИВЗ (n = 60).
Иммунохимические исследования слюны проводили среди детей подгрупп 1 (n = 11) и 2 (n = 13), а также взрослых подгрупп 3 (n = 18) и 4 (n = 7).
Критерии включения:
- – наличие диагноза, поставленного впервые или имевшегося в анамнезе;
- – информированное согласие пациента или его родителей на участие в исследовании;
- – отсутствие тяжелых психических и соматических заболеваний.
Критерии исключения:
- – участие в иных медицинских исследованиях;
- – прием иммунотропных препаратов.
Мазки производили стерильными ватными тампонами («Nuova Aptaca», Италия) с задней стенки глотки и поверхности миндалин, тампоны помещали в транспортную среду Эймса и доставляли в лабораторию, где проводили посевы на чашки с селективными средами. Подробное описание приведено в нашем предыдущем исследовании [3].
Совокупную активность АМП оценивали, используя ранее разработанный метод, в основу которого положено свойство АМП нарушать целостность цитоплазматической мембраны микроорганизмов [35]. Метод модифицирован путем использования в качестве порообразующих агентов низкомолекулярных фракций слюны, а также путем исключения стадии микроскопии и введения стадии спектрофотометрии, что значительно упростило методику и повысило ее точность.
Слюну собирали в пластиковые контейнеры и замораживали порциями при -25 °С. Перед проведением анализа слюну размораживали и отделяли от макрочастиц путем центрифугирования при 16 000 об/мин в течение 5 мин. Получение низкомолекулярной фракции проводили путем центрифугирования при 16 000 об/мин в течение 10 мин с использованием фильтрующих насадок «Amicon ultra» («Merсk», Германия) с размером пор 100 кДа.
В качестве модельного микроорганизма использовали дрожжи Candida albicans № 927 из коллекции Лаборатории физиологии грибов и бактерий ФГБНУ «НИИВС им. И.И. Мечникова». Для приготовления клеточной суспензии использовали 20-часовую культуру Candida albicans № 927. Аликвоты образцов слюны соединяли с суспензией клеток дрожжей и инкубировали при +32 °С на шейкере в течение 2 ч, после чего центрифугировали в течение 5 мин со скоростью 16 000 об/мин. Полученный осадок инкубировали с 300 мкл красителя бромкрезолового пурпурного, pH 4,6 в тех же условиях, клетки отделяли центрифугированием в течение 5 мин со скоростью 16 000 об/мин. Супернатант разбавляли калий-фосфатным буфером (pH 4,6) в 50 раз и измеряли оптическую плотность на спектрофотометре «Genesys 10S UV-Vis» при длине волны 440 нм. Активность АМП выражали как процент красителя, поглощенного убитыми клетками тест-культуры C. albicans, по следующей формуле:

ОПконтр. – ОП в контрольном варианте с физраствором вместо слюны;
ОПопыт. – ОП в варианте со слюной.
Для определения концентрации секреторного IgA (sIgA) в слюне использовали набор «IgA секреторный-ИФА-БЕСТ» («Вектор-БЕСТ», Россия). Исследуемые образцы, согласно инструкции, использовали в разведении 1:2000. Об уровне IgG-антител судили по величине оптической плотности (ОП), которую измеряли на спектрофотометре «ЭФОС 9305» (Россия) при длине волны 450 нм через 2–3 мин после внесения стоп-реагента.
 Для определения типа распределения полученных выборок использовали такие методы первичной статистической обработки, как среднее и медиана. При обработке малых выборок (менее 16 объектов, при котором t-распределение начинает существенно отличаться от нормального) использовали U-тест Манна–Уитни для двух независимых выборок. Для исследования взаимосвязи между двумя независимыми выборками применяли корреляционный анализ.
Для определения типа распределения полученных выборок использовали такие методы первичной статистической обработки, как среднее и медиана. При обработке малых выборок (менее 16 объектов, при котором t-распределение начинает существенно отличаться от нормального) использовали U-тест Манна–Уитни для двух независимых выборок. Для исследования взаимосвязи между двумя независимыми выборками применяли корреляционный анализ.
Результаты
В нашем предыдущем исследовании был подробно рассмотрена структура нозологических форм РИВЗ. Однако при разбивке на взрослую и детскую группы выяснилось, что в детской отсутствовали пациенты с ХОБЛ, а во взрослой – с ОНР и АТ (рис. 1).
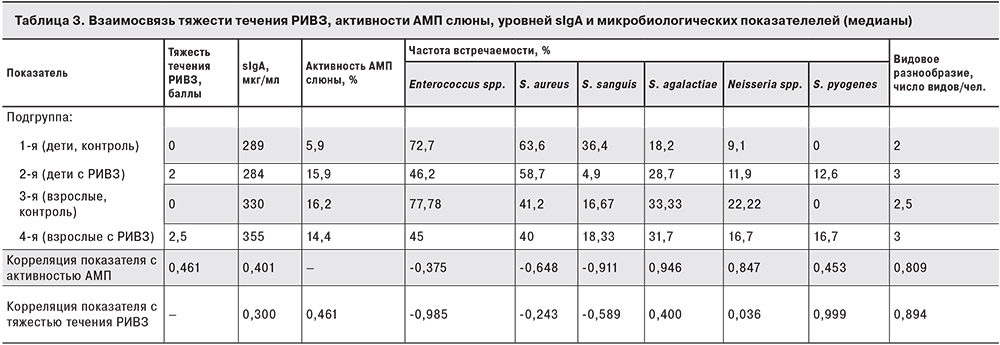
Распределение пациентов по тяжести течения РИВЗ представлено на рис. 2: видно, что среди детей процент пациентов со средней степенью тяжести РИВЗ (2 балла) значительно выше, чем среди взрослых.
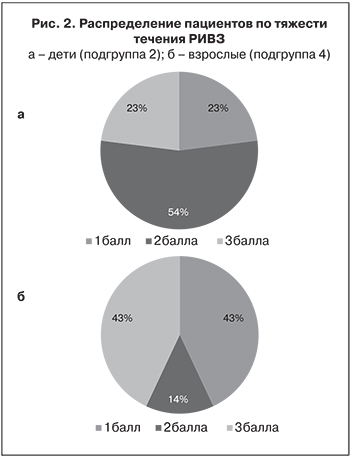
Данные об активности АМП фракции слюны, рассчитанные по вышеуказанной формуле, представлены на рис. 3. В подгруппах 1 и 3 отмечена меньшая активность АМП, чем в подгруппах 2 и 4. Установлено, что показатели активности АМП у пациентов с РИВЗ были в 1,1–3,0 раза выше, чем у здоровых участников исследования.
Особенно четко видно различие у детей: в подгруппе 2 (медиана 15,9%) наблюдали 3-кратное увеличение активности АМП по сравнению с ее значениями в подгруппе 1 (медиана 5,9%). Не такая ясная ситуация наблюдалась среди взрослых. Вследствие небольшой выборки и большого разброса показателей в подгруппе 4 (n = 7) превышение активности АМП было не таким значительным. Различия значений в подгруппе 1 по сравнению с подгруппами 2 и 3, оцененные по критерию Манна–Уитни, значимы (p ≤ 0,01), а по сравнению с подгруппой 4 значимы в интервале 0,01 ≤ p ≤ 0,05; различия значений между подгруппами 2 и 3, а также 2 и 4 незначимы (p > 0,05). При сравнении медиан значений активности АМП и тяжести течения РИВЗ по всем указанным выборкам отмечена слабая положительная корреляция (табл. 3).
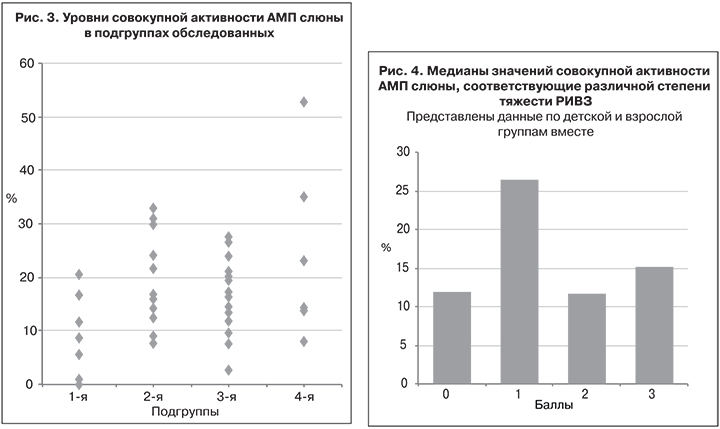
Расположив значения тяжести течения РИВЗ по возрастанию баллов от 0 до 3 против соответствующих значений активности АМП, получим гистограмму, представленную на рис. 4. Наибольшее различие имело место между 1-й степенью тяжести и остальными, достоверность чего подтверждена статистически (p ≤ 0,01), тогда как активность АМП при 2-й и 3-й степени тяжести достоверно не отличалась от контрольных показателей (p > 0,05). Объяснить этот факт можно тем, что легкая степень РИВЗ связана со способностью организма вырабатывать достаточное количество активных АМП, тогда как осложнение течения РИВЗ обусловлено неспособностью организма адекватно реагировать на инфекцию. В связи с такой неоднозначной зависимостью между тяжестью течения РИВЗ и активностью АМП слюны корреляция между этими показателями по всей выборке в целом совсем низкая – r = 0,273.
 Обсуждение
Обсуждение
В связи с защитой слизистых оболочек влагалища в литературе упоминаются следующие пептиды: лактоферрин, кальпротектин, лизоцим, секреторный ингибитор лейкопротеазы, кателицидины и дефензины. В более раннем нашем исследовании была изучена взаимосвязь между активностью АМП влагалища и наличием бактериального вагиноза и кандидозного вульвовагинита [35]. Мы использовали метод, основанный на сборе вагинального отделяемого и взаимодействии его с тест-культурами C. albicans в случае кандидозного вульвовагинита и E. coli – в случае бактериального вагиноза [34] в собственной модификации. При обоих патологических состояниях установлена обратная корреляция высокой степени между совокупной активностью АМП и тяжестью течения заболевания. Очевидно, что совокупная активность АМП зависит от изучаемого локуса и нозологии.
Помимо АМП прямой антимикробной активностью обладают также секреторные иммуноглобулины [36]. В слюне человека преобладающим классом является sIgA [37]. В этой связи было целесообразно определить уровни этих антител в нефракционированной слюне (см. табл. 3). Полученные результаты согласуются с данными A.J. Macpherson и соавт. [38], которые показали, что уровень sIgA в слюне с возрастом повышается. Однако значения sIgA в изученных выборках достоверно не различались (p > 0,05), а сколько-нибудь значимой корреляции медиан показателя в подгруппах с тяжестью течения РИВЗ/активностью АМП не обнаружено. В литературе есть данные о прогностической значимости этого показателя в оценке состояния здоровья населения при массовых обследованиях [39]. Авторы указывают, что снижение sIgА может свидетельствовать о недостаточности функции местного иммунитета, а его повышенный уровень – о дисбалансе в иммунной системе. По полученным нами данным такого вывода сделать нельзя.
Количество видов микроорганизмов, обнаруживаемых в мазках из ротоглотки у одного человека, – видовое разнообразие – варьировало среди детей от 1 до 4 (подгруппа 1) и от 1 до 6 (подгруппа 2), среди взрослых в подгруппах 3 и 4 – также от 1 до 5. Медианы этих показателей представлены в табл. 3. Важно отметить, что видовое разнообразие имело положительную корреляцию высокой силы с тяжестью течения РИВЗ и активностью АМП слюны, но практически не было связано с уровнем sIgА (r = 0,364).
Можно попытаться привести такие разные показатели антимикробной защиты, как активность АМП и уровни sIgА, к общему знаменателю и вычислить их суммарную активность. Для этого выразим каждое значение показателей из табл. 3 в процентах, принимая за 100% наименьший показатель в столбце. После этого сложим полученные числа построчно и получим следующие значения показателей «интегральной» защиты: подгруппа 1 – 202%, подгруппа 2 – 370%, подгруппа 3 – 391% и подгруппа 4 – 369%. Видно, что минимальная антимикробная защита слюны имеет место у здоровых детей, а максимальная – у здоровых взрослых.
Для оценки взаимосвязи между наиболее часто встречающимися видами микроорганизмов, тяжестью течения РИВЗ и активностью АМП слюны мы расположили виды в порядке убывания частоты встречаемости в подгруппе 1. Оказалось, что частота встречаемости одних видов (Enterococcus spp., S. aureus и S. sanguis) имела отрицательную и в основном высокую корреляцию с тяжестью течения РИВЗ и активностью АМП, тогда как остальные виды (S. agalactiae, Neisseria spp. и S. pyogenes) – положительную и в половине случаев сильную корреляцию с указанными показателями. Видно, что с тяжестью течения РИВЗ наиболее сильно связана встречаемость S. pyogenes, что свидетельствует о наиболее выраженной условной патогенности этих бактерий, тогда как наличие Enterococcus spp., напротив, характерно для здоровых носителей и в гораздо меньшей степени присуще пациентам с РИВЗ. С активностью АМП наиболее сильно коррелировала встречаемость S. agalactiae и Neisseria spp., а значительная обратная взаимосвязь наблюдалась с встречаемостью видов S. aureus и S. sanguis. Важно отметить, что золотистый стафилококк встречался у детей в 1,5 раза чаще, чем у взрослых.
Выводы
- Значения совокупной активности АМП слюны у здоровых детей были достоверно ниже, чем у детей и взрослых с РИВЗ, а также здоровых взрослых.
- Наибольшая активность АМП слюны имела место при легком течении РИВЗ (медиана 26%) и достоверно отличалась от таковой у здоровых добровольцев (медиана 11%) и пациентов со среднетяжелой и тяжелой формами РИВЗ (медианы 11 и 15%) (p ≤ 0,01).
- Уровни sIgA (медианы) у детей были несколько ниже, чем у взрослых, однако достоверно не различались (p > 0,05), а значимой корреляции между медианами показателя в подгруппах и тяжестью течения РИВЗ/активностью АМП не обнаружено.
- Разнообразие видов микроорганизмов, обнаруженных в ротоглотке (медианы), имело положительную корреляцию высокой силы с тяжестью течения РИВЗ (r = 0,894) и активностью АМП слюны (r = 0,809), но не было связано с уровнем sIgА (r = 0,364).
- Судя по значительно бóльшей частоте встречаемости среди здоровых людей, чем среди пациентов с РИВЗ, а также высокой обратной корреляции между встречаемостью и тяжестью течения РИВЗ (r = -0,985), бактерии рода Enterococcus spp. являются нормальными обитателями ротоглотки. Вид S. pyogenes, исходя из прямо противоположных показателей, напротив, можно отнести к наиболее оппортунистическим видам, выделяемым при РИВЗ.
- Вид S. aureus в 1,5 раза чаще обнаруживали у детей, чем у взрослых, причем значимой корреляции между тяжестью течения РИВЗ и встречаемостью S. aureus не обнаружено (r = - 0,243).


