В процессе системного применения антиретровирусной терапии (АРТ) время от времени встает вопрос: что целесообразнее – создание антиретровирусных препаратов или новых фармакологических групп с инновационным механизмом действия, либо разработка и внедрение дженерических (воспроизведенных) уже хорошо известных лекарств [1]?. Говоря об общепринятых подходах к применению воспроизведенных препаратов, необходимо подчеркнуть, что, например, по мнению экспертов ВОЗ, использование таких препаратов в лечении ВИЧ-инфекции является одной из стратегических целей на пути расширения доступа к медицинской помощи [2]. Во-первых, это позволит значительно снизить прямые затраты на лечение, более широко применять данные препараты в странах с ограниченными ресурсами. Во-вторых, нельзя забывать, что сроки патентной защиты инновационного препарата зависят от патентного законодательства страны, где он выпускается. Так, в США, где производится большинство антиретровирусных препаратов, патентная защита действует 12 лет. После окончания срока действия патента любая фармакологическая фирма может начать воспроизводить препарат и выходить с ним на рынок. Несомненно, одним из достоинств воспроизведенных препаратов является их низкая цена в связи с отсутствием затрат на разработку, клинические исследования, лицензионные выплаты и т. д. [3]. И наконец, важно учитывать тот факт, что эти препараты имеют длительный опыт практического применения.
Традиционно медицинская общественность, пациентские сообщества и правозащитные организации настороженно относятся к таким заменам оригинальных препаратов в клинической практике, наделяя дженерики всеми возможными и придуманными отрицательными характеристиками: плохая переносимость, большое количество нежелательных явлений (НЯ) либо побочных эффектов, большое число таблеток или капсул на прием, минимальный экономический эффект и др. [4, 5]. В некоторых случаях для такого скептического отношения имеются основания. Действительно, результаты некоторых сравнительных исследований, личный опыт врачей, отзывы пациентов свидетельствуют о том, что новые воспроизведенные препараты иногда не выдерживают никакого сравнения с оригинальными (референтными) [6].
В связи с нынешней эпидемической ситуацией в Российской Федерации, когда происходит быстрое распространение ВИЧ-инфекции, постоянное накопление новых зарегистрированных случаев инфицирования людей, большинство из которых уже при выявлении нуждаются в назначении АРТ [7], остро встает вопрос о расширении охвата специфическим лечением, особенно терапией первой линии, все большего количества пациентов [8, 9]. Фармакоэкономический анализ показывает, что при нынешних темпах расширения назначения АРТ крайне сложно рассчитывать на то, что в ближайшие годы удастся взять эпидемический процесс под контроль [10, 11].
 Одним из путей решения данной проблемы является активное внедрение в практику лечения пациентов с ВИЧ-инфекцией новых доступных эффективных и безопасных лекарственных препаратов [12–15]. Одним из них как раз и является дизаверокс (AZT/3TC) – комбинация ламивудина (150 мг) и зидовудина (300 мг) – воспроизведенный аналог референтного препарата комбивир (AZT/3TC), зарегистрированный в Российской Федерации и разрешенный для лечения пациентов [16].
Одним из путей решения данной проблемы является активное внедрение в практику лечения пациентов с ВИЧ-инфекцией новых доступных эффективных и безопасных лекарственных препаратов [12–15]. Одним из них как раз и является дизаверокс (AZT/3TC) – комбинация ламивудина (150 мг) и зидовудина (300 мг) – воспроизведенный аналог референтного препарата комбивир (AZT/3TC), зарегистрированный в Российской Федерации и разрешенный для лечения пациентов [16].
Актуальность исследования заключалась в сравнении данных препаратов, оценке получаемых результатов по преодолению иммуносупрессии, вирусной активности, скорости и степени достижения того или иного эффекта. Крайне важно было получить системные представления о частоте и характере НЯ, которые могут развиться на ранних и отдаленных этапах проводимой АРТ [17].
Цель исследования – сравнительная оценка эффективности и безопасности препаратов AZT/3TC (дизаверокс) и AZT/3TC (комбивир) в сочетании с препаратом лопинавир/ритонавир (LPV/r – калетра) у взрослых пациентов с ВИЧ-инфекцией, которые ранее не получали лечения, в течение 24 недель АРТ, в том числе проведение анализа иммунологической и вирусологической эффективности и сравнение частоты развития НЯ.
Материалы и методы
На базе СПб ГБУЗ «Центр по профилактике и борьбе со СПИД и инфекционными заболеваниями» проведено одноцентровое открытое рандомизированное проспективное исследование IV фазы по изучению сравнительной эффективности и безопасности воспроизведенного и референтного препаратов AZT/3TC.
Критерии включения:
- ВИЧ-инфекция, подтвержденная методами ИФА и ИБ.
- Возраст пациентов 18–50 лет.
- Отсутствие беременности у женщины на момент включения в исследование и на протяжении последующих 6 мес.
- ВИЧ-инфекция в стадии 3, 4А, 4Б по классификации В.И. Покровского.
- Количество CD4+-лимфоцитов в крови от 200 до 500 клеток/мкл.
- Уровень РНК ВИЧ (ПЦР) более 1000 копий/мл.
- Ремиссия по употреблению ПАВ (наркотики, алкоголь) не менее 3 мес.
- При наличии хронических гепатитов С и/или В уровень трансаминаз (АЛТ, АСТ) не должен превышать верхних границ нормы более чем в 3 раза.
- Подписанное до начала исследования информированное согласие.
- Пациент способен проглатывать таблетки, не разжевывая их.
- Пациент имеет мотивацию к началу АРТ (заключение психолога: «приверженность к АРТ к стадии формирования или сформирована»).
Критерии исключения:
- Невозможность получения информированного согласия.
- Возраст моложе 18 и старше 50 лет.
- Количество СD4+-лимфоцитов < 200 и > 500 клеток/мкл.
- Активный вирусный гепатит (уровень АЛТ и АСТ, превышающий норму более чем в 3 раза) либо цирротическая стадия гепатита.
- Опыт применения АРТ (в том числе с целью профилактики вертикальной передачи).
- Активное употребление ПАВ (ремиссия менее 3 мес.).
- Клинически значимые сопутствующие заболевания: сердечная недостаточность, туберкулез, почечная и печеночная недостаточность, психические заболевания и т. д.
- Онкологические заболевания (лучевая или химиотерапия).
- Любое состояние, которое может повлиять (по мнению Исследователя) на прием препаратов.
- Тяжелый иммунодефицит (стадия 4В), тяжелые оппортунистические инфекции (в том числе токсоплазмоз головного мозга, генерализованная ЦМВ-инфекция, криптококковый менингит и другие острые заболевания ЦНС).
- Лабораторные показатели во время скрининга: уровень АЛТ и АСТ, превышающий норму более чем в 3 раза; уровень креатинина и мочевины в 3 и более раз выше нормы; уровень гемоглобина менее 120 г/л; уровень нейтрофилов менее 1,5 х 109/л; тяжелая тромбопения (менее 50 х 109/л).
Среди ВИЧ-инфицированных больных обоего пола, не получавших ранее АРТ, в соответствии с критериями включения/невключения был отобран 61 человек. Пациенты были разделены на 2 группы методом случайной выборки. Группы были сопоставимы по всем параметрам. Пациенты 1-й группы принимали воспроизведенный препарат AZT/3TC и LPV/r, пациенты 2-й группы – референтный AZT/3TC и LPV/r.
Критериями безопасности и переносимости антиретровирусных препаратов являлось наличие НЯ и серьезных НЯ (СНЯ), а также оценка лабораторных показателей (общего и биохимического анализов крови).
Количество CD4+-лимфоцитов в крови определяли методом проточной цитофлюорометрии по одноплатформенной технологии на проточном цитометре FACSСalibur («Becton Dickinson», США).
Вирусную нагрузку ВИЧ определяли с помощью тест-систем Abbott RealTime HIV-1 («Abbott», США) и COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version 2.0.
Достоверность полученных результатов была подтверждена при использовании современной компьютерной системы STATISTICA for Windows (версия 5.5, лиц. № AXXR402C29502 3FA) критериев сравнения параметрических и непараметрических показателей.
Сравнение параметров в группах исследования проводили с помощью критериев Манна–Уитни, Колмогорова–Смирнова, медианного c2 и модуля ANOVA. Оценку частот выполняли посредством критериев c2, Пирсона, Фишера. Анализ динамики показателей проводили с помощью критерия знаков и Т-критерия Вилкоксона. Различия считали статистически достоверными при р < 0,05.
Согласие каждого пациента на участие в исследовании было подтверждено подписанием ими формы информированного согласия в двух экземплярах.
Результаты и обсуждение
Все пациенты состояли на диспансерном учете в СПб ГБУЗ «Центр по профилактике и борьбе со СПИД и инфекционными заболеваниями» Характеристика пациентов представлена в табл. 1.
Оценка эффективности. Иммунологическую и вирусологическую эффективность оценивали на 12-й и 24-й неделе лечения.
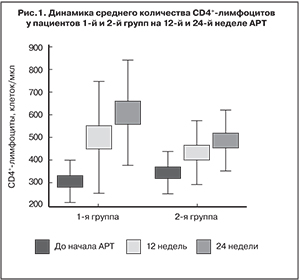
В обеих группах наблюдалась хорошая иммунологическая эффективность (рис. 1).
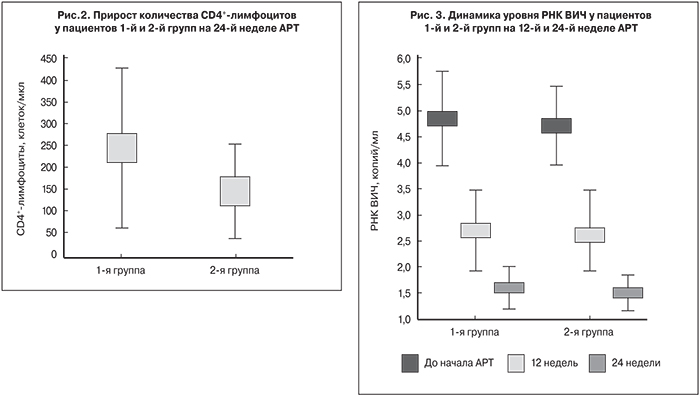
 Вместе с тем, у пациентов 1-й группы прирост количества CD4+-лимфоцитов был достоверно выше, чем у пациентов 2-й группы (р < 0,05): медиана прироста составила 230 и 144,5 клеток/мкл соответственно (рис. 2).
Вместе с тем, у пациентов 1-й группы прирост количества CD4+-лимфоцитов был достоверно выше, чем у пациентов 2-й группы (р < 0,05): медиана прироста составила 230 и 144,5 клеток/мкл соответственно (рис. 2).
Обе схемы продемонстрировали высокую вирусологическую эффективность, которая была оценена по степени снижения вирусной активности ВИЧ. Средний уровень РНК ВИЧ за 24 недели лечения у пациентов 1-й группы снизился с 5,4 до 2,3 log, у пациентов 2-й группы – с 5,2 до 1,7 log (рис. 3).
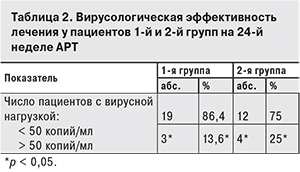
Доля пациентов с подавленной вирусной нагрузкой (< 50 копий/мл) к исходу 24-й недели лечения в 1-й группе оказалась достоверно выше, чем во 2-й (р < 0,05) (табл. 2).
Оценка безопасности. Безопасность и переносимость антиретровирусных препаратов определяли по наличию НЯ и СНЯ, а также по лабораторным показателям [18].
НЯ оценивали через 4, 12 и 24 недели лечения, они были зафиксированы у 51 (85%) пациента. За время исследования СНЯ не зарегистрировано.
Частым побочным явлением в обеих группах была анемия. В 1-й группе она развилась у 5 пациентов на 14–30-й день приема препаратов. У 4 пациентов анемия была средней степени тяжести. Это потребовало отмены воспроизведенного препарата AZT/3TC, после чего анемия разрешилась. У 1 пациента анемия была легкой степени, связь ее с приемом воспроизведенного AZT/3TC – вероятная, отмены препарата не потребовалось. Во 2-й группе анемия была выявлена у 9 пациентов. У 1 она была железодефицитной в связи с носовыми кровотечениями и не связана с АРТ. Анемия начинала развиваться на 14–69-й день АРТ и, вероятно, была связана с приемом референтного препарата AZT/3TC. У 4 пациентов она была средней тяжести и потребовала замены препарата. У других четверых пациентов анемия легкой степени не требовала никаких вмешательств. Лечение по прежней схеме было продолжено [19, 20].
Самым частым побочным явлением было небольшое повышение уровня холестерина (1-й группе – у 8 пациентов, во 2-й – у 15), которое во всех случаях, вероятно, было связано с приемом препарата LPV/r и не требовало никаких вмешательств.
Также достаточно часто наблюдали диарею: в 1-й группе – у 8 пациентов, во 2-й – у 10. Данное НЯ в обеих группах носило легкий характер, было, вероятно, связано с приемом препарата LPV/r (калетра) и не требовало отмены АРТ. В 1-й группе 2 пациента в качестве сопутствующей терапии принимали панкреатин, еще 2 – лоперамид. Диарея, как правило, начиналась в 1-й день приема и завершилась к 20–120-му дню лечения. Во 2-й группе 6 пациентов принимали сопутствующую терапию – лоперамид и панкреатин. У 8 пациентов этой группы диарея завершилась на 18–90-й день, у 2 пациентов она сохраняется.
Тошноту наблюдали у 2 пациентов 1-й группы: у одного – легкой степени тяжести, у другого – средней. Она возникла на 1-й и 15-й день терапии, была, вероятно, связана с приемом воспроизведенного препарата AZT/3TC и завершились на 56-й и 120-й день самостоятельно. Во 2-й группе тошноту наблюдали у 7 пациентов, у 3 из них она сопровождалась рвотой. У всех 7 пациентов тошнота и рвота были, вероятно, связаны с приемом референтного препарата AZT/3TC и возникли на 1–2-й день приема. У 4 пациентов эти явления были средней степени тяжести и потребовали отмены препарата.
Лейкопения легкой степени была зарегистрирована у 4 пациентов 1-й группы. У 1 она была средней степени и потребовала, вместе с анемией, отмены воспроизведенного препарата. У 3 других лейкопения носила легкий характер, развивалась на 14–30-й день приема, не требовала дополнительных вмешательств, ее связь с приемом AZT/3TC – вероятная. Во 2-й группе также у 4 пациентов развилась лейкопения легкой степени, связь с приемом референтного препарата AZT/3TC – вероятная, дополнительных вмешательств не требовалось [21].
Эритроцитопения легкой степени была зарегистрирована в 1-й группе у 2 пациентов и развилась на 90–180-й день; во 2-й группе – также у 2 пациентов, развилась на 30–90-й день. В обеих группах связь эритроцитопении с приемом воспроизведенного и референтного препаратов была вероятной и не требовала дополнительных вмешательств.
В 1-й группе повышение уровня трансаминаз произошло у 4 пациентов, у 1 – средней степени тяжести, связь с приемом AZT/3TC была расценена как вероятная, и препарат был заменен. У остальных 3 пациентов это НЯ носило легкий характер и не было связано с приемом препарата. Во 2-й группе оно было выявлено также у 4 пациентов: у 2 – легкой и у 2 – средней степени тяжести. У 3 пациентов вероятна связь с приемом референтного препаратов AZT/3TC .
В 1-й группе у 1 пациента наблюдалось повышение уровня амилазы легкой степени, вероятно, связанное с приемом AZT/3TC. Повышение уровня глюкозы легкой степени отмечено у 1 пациента в 1-й группе и у 1 – во 2-й. В обоих случаях имела место вероятная связь с приемом препарата LPV/r.
Во 2-й группе 2 пациента отмечали легкое вздутие живота, что, вероятно, связано с приемом LPV/r. В этой же группе 1 пациент жаловался на неприятный вкус во рту. Данное НЯ, возможно, связано с приемом референтного препарата AZT/3TC.
Повышение уровня глюкозы в крови (G1) наблюдалось у 1 пациента в каждой группе. У пациента 2-й группы врачи-исследователи связали его с приемом LPV/r. АРТ прервали из-за НЯ в 1-й группе 5 (15,6%) пациентов, во 2-й — 8(27,6%).
Таким образом, при использовании обеих схем АРТ были получены сопоставимые иммунологические и вирусологические показатели эффективности препаратов. Тем не менее, медиана прироста CD4+-лимфоцитов в крови у пациентов 1-й группы и 2-й групп составила 230 и 144,5 клеток/мкл соответственно (р < 0,05). Доля пациентов с подавленной вирусной нагрузкой на 24-й неделе лечения в 1-й группе оказалась достоверно выше, чем во 2-й (р < 0,05).
Из НЯ наиболее часто наблюдали анемию, лейкопению, диарею, тошноту. Доля пациентов, прервавших схему АРТ из-за НЯ, в 1-й группе.была достоверно ниже.
Полученные результаты говорят о том, что отечественный воспроизведенный препарат AZT/3TC по своим качествам не уступает (терапевтически эквивалентен) референтному препарату.


