Сохраняющийся на протяжении многих лет интерес исследователей и практикующих врачей различных специальностей к герпесвирусным инфекциям (ГВИ) не является случайным. Герпесвирусы пантропны и широко распространены в человеческой популяции.
Все герпесвирусы используют стратегию длительного латентного существования в организме хозяина, которая периодически прерывается литической реактивацией части латентных клеток с образованием свободных вирионов, передающихся здоровым людям. Кроме того, высокая мутационная активность вирусного генома способствует ускользанию вируса от иммунологического контроля, индуцируя латентную, острую и хроническую формы инфекции, ухудшающие качество жизни пациента [1–2].
По данным вирусологических, серологических исследований, результатам исследований методом полимеразной цепной реакции (ПЦР), к исходу 3-го года жизни более 80% детей инфицированы различными видами герпесвирусов. У школьников частота инфицирования составляет около 90%, у взрослых – более 90% [3, 4]. По данным ВОЗ, до 95% населения планеты инфицированы одним или несколькими штаммами вируса. В настоящее время выделено 8 патогенных для человека вирусов семейства Herpesviridae: вирус простого герпеса 1-го (ВПГ-1) и 2-го (ВПГ-2) типов, Varicella-Zoster (VZV), цитомегаловирус (ЦМВ, CMV), вирус Эпштейна–Барр (ВЭБ, EBV), вирус герпеса человека 6-го (ВГЧ-6), 7-го (ВГЧ-7) и 8-го (ВГЧ-8) типов.
У детей спектр клинических проявлений ГВИ весьма разнообразен: он зависит от локализации патологического процесса, его распространенности, состояния иммунной системы больного и антигенного типа вируса [1, 4–10].
Верификация ГВИ в настоящее время основывается на прямых и непрямых методах лабораторной диагностики: детекции ДНК вирусов методом ПЦР и/или их антигенов в реакции иммунофлюоресценции; выявлении специфических IgG, IgМ, IgA с определением авидности IgG-антител в иммуноферментном анализе (ИФА), а также наличия IgМ и IgG к отдельным маркерным белкам герпесвирусов в иммуноблоте или иммунопреципитации. Культуральный метод (с изоляцией вируса на чувствительных клетках in vitro и последующей идентификацией на специальном оборудовании) используется в исследовательских лабораториях.
ПЦР является «золотым стандартом» диагностики ГВИ, его используют для детекции всех восьми типов герпесвирусов, патогенных для человека. Недостатком классической ПЦР являются ложноположительные реакции из-за загрязнения посторонней ДНК или субоптимального праймер-матричного соотношения. В настоящее время существует метод, лишенный вышеперечисленных недостатков, – это метод ПЦР в реальном времени (REAL–Time PCR). Сущность метода заключается в исследовании накопления продуктов амплификации с помощью специального прибора, особенностью которого является возможность детектировать процесс флюоресценции в каждом цикле амплификации без последующего электрофореза, что позволяет максимально снизить риск контаминации продуктами ПЦР и уменьшить число ложноположительных результатов. Таким образом, метод REAL–Time PCR, в отличие от классической ПЦР, позволяет количественно определить отношение ДНК к РНК инфекционных агентов в исследуемом материале [5, 11]. До настоящего времени не достигнута стандартизация метода, в разных лабораториях используют праймеры на различные регионы вирусного генома и разные протоколы исследования. Существует мнение, что цельная кровь является наиболее подходящим биологическим материалом для диагностики активной ГВИ, тогда как выявление вирусной ДНК в других биологических субстратах может свидетельствовать о латентной инфекции. Поэтому наиболее значимой диагностической ценностью обладает выявление генома герпесвирусов в крови, в то время как выделение антигена вирусов в слюне и моче регистрируется и у здоровых людей. Данная точка зрения не может рассматриваться однозначно, поскольку выявление ДНК вируса и в других субстратах (не в крови) не исключает медленного течения инфекции, в результате которого клиническая манифестация поражения того или иного органа может проявиться спустя достаточно продолжительное время. В связи с этим любая идентификация вируса, независимо от субстрата, должна обязательно быть дополнена серологическим исследованием [11, 12].
Метод ИФА используется наиболее часто и позволяет установить стадию инфекции. Он имеет большое значение с учетом того, что выделение вируса непостоянно и зависит от типа вируса, формы заболевания и реактивности организма, в то время как наличие антител в крови остается пожизненно. Метод ИФА основан на обнаружении в венозной крови пациентов антител иммуноглобулинов класса М и G (IgM и IgG). При «свежем инфицировании» вначале появляются IgM, достигая максимума к двум месяцам, затем происходит снижение на фоне появления и увеличения IgG. При рецидиве инфекции (реактивации) возможно появление IgM на фоне увеличения титра IgG. Определение только антител с подсчетом их титров в сыворотке крови в момент забора не является основанием для установления диагноза активного процесса. Недостатком ИФА является то, что в условиях угнетения иммунного ответа (что имеет место у больных системными, аутоиммунными заболеваниями, а также у пациентов, получающих иммуносупрессивную терапию) антителогенез может быть нарушен. Также чем меньше возраст ребенка, тем ниже титры антител по данным ИФА. Кроме того, нельзя не учитывать, что некоторые ГВИ (в частности, ЦМВ и ВЭБ) относятся к инфекциям с нетипичной динамикой антителообразования, когда наличие IgМ не является достоверным и достаточным признаком для дифференциации стадии заболевания [11]. Возможно также выявление перекрестно реагирующих антител к другим ДНК-вирусам. Для установления точного момента инфицирования и разграничения первичной реинфекции и реактивации инфекционного процесса используют тест на определение авидности IgG-антител. Уровень авидности пропорционален дозе и природе антигена, а также индивидуальному уровню соматических мутаций. Низкие дозы антигена приводят к более быстрому возрастанию авидности, а высокие – к более медленному. Наличие высокоавидных антител IgG говорит о вторичном иммунном ответе в случае реинфекции или реактивации, также они могут определяться в более низких титрах при латентной фазе процесса [4, 12].
Все герпесвирусы являются ДНК-содержащими, сходны по морфологии, типу нуклеиновой кислоты, способу репродукции в ядрах инфицированных клеток. Так, β-герпесвирусы (ЦМВ, ВГЧ-6 и ВГЧ-7) отличаются выраженным цитопатическим эффектом, длительным циклом репликации. В то же время вирус может приводить к подавлению эффекторных функций (например, экспрессии цитокинов) и апоптозу инфицированных клеток у лиц с ослабленным иммунитетом. В свою очередь γ-герпесвирусы, включая ВЭБ, характеризуются тропизмом к В- и Т-лимфоцитам, лимфоидным клеткам, в которых они способны размножаться и длительно персистировать. ВПГ-1 нарушает механизмы, связанные с презентацией антигена Т-лимфоцитам, поэтому последние не распознают инфицированные клетки как чужеродные, что позволяет «избегать» влияния на них СD8+-лимфоцитов, и патологический процесс приобретает вялотекущий характер. В отличие от последних ВЭБ не блокирует экспрессию главного комплекса гистосовместимости (англ. major histocompatibility complex – MHC) I класса. Его мишенями являются В-лимфоциты. Последние, трансформированные ВЭБ, экспрессируют большее число белков HLA I класса по сравнению с неинфицированными клетками, что защищает клетки-мишени, пораженные вирусом, от лизиса НК-клетками (натуральными киллерами) и приводит к персистированию инфекции, возможности формирования длительной иммуносупрессии. С другой стороны, персистирование ВЭБ стимулирует выработку специфических анти-ЕВV-антител В-лимфоцитами, что в условиях недостаточности клеточного звена иммунного ответа может способствовать (в силу гомологичности белковых компонентов ЕВV с таковыми HLA I класса) активации аутоиммунных процессов. Это позволяет предположить, что ГВИ служит не только провоцирующим фактором развития дебюта ювенильного идиопатического артрита (ЮИА), но и может являться одним из патогенетических звеньев развития патологического процесса [13, 14].
В отечественной и зарубежной литературе активно обсуждается роль ГВИ в формировании соматической патологии [15–23], в том числе как триггерного и/или патогенетического фактора, приводящего к развитию аутоиммунных процессов [14, 24–28].
Возбудители инфекций рассматриваются как возможные этиологические факторы таких аутоиммунных заболеваний, как системная красная волчанка (СКВ), ревматоидный артрит (РА), рассеянный склероз (РС), синдром Шегрена, сахарный диабет 1-го типа (СД1). Описано несколько механизмов, благодаря которым инфекционные агенты могут приводить к развитию аутоиммунного процесса: поликлональная активация В-лимфоцитов, дефицит CD8+-лимфоцитов, молекулярная мимикрия, модификация клеточных антигенов, активация суперантигена, адъювантный эффект [13]. По данным зарубежной литературы, наиболее изучена взаимосвязь аутоиммунных реакций с γ-герпесвирусами, в частности, с ВЭБ и ЦМВ, поскольку данные вирусы характеризуются тропизмом к лимфоидным клеткам (Т- и В-лимфоцитам). Исследования, связанные с изучением серологических показателей и/или вирусной нагрузки, также указывают на потенциальную роль этих вирусов в развитии аутоиммунных заболеваний, включая РА [29–30], СКВ [31], РС [32], СД1 [30].
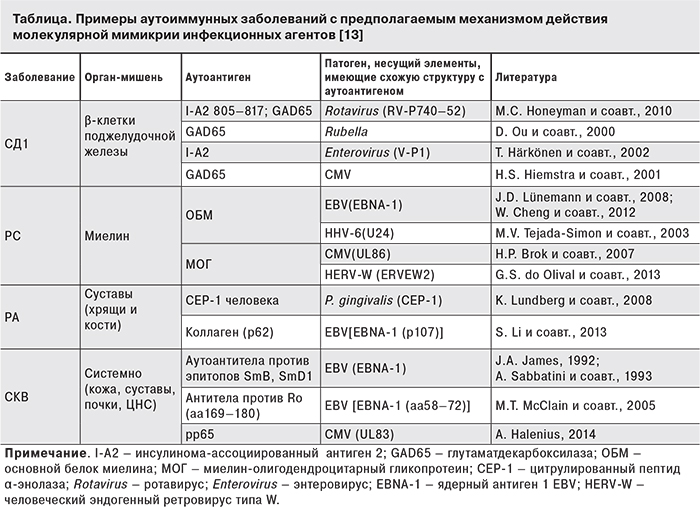
Наиболее убедительной моделью развития аутоагрессии в настоящее время является гипотеза молекулярной мимикрии, появившаяся около 20 лет назад. Молекулярная мимикрия может инициировать аутоиммунизацию в результате развития перекрестной иммунной реакции при структурном сходстве антигенных детерминант вируса и хозяина либо в результате модификации антигенов тканей организма (см.таблицу).
Однако все больше появляется свидетельств в пользу того, что одной лишь молекулярной мимикрии недостаточно для развития аутоиммунного процесса. Широко распространено мнение о том, что суперантигены вносят значительный вклад в развитие аутоиммунитета. Суперантигены представляют собой группу патогенных бактериальных и вирусных белков, которые способны активировать большое количество Т- и В-лимфоцитов, независимо от антигенной специфичности этих клеток. Отличительной особенностью суперантигенов является их способность активировать лимфоциты без необходимой предварительной презентации на поверхности антигенпрезентирующих клеток (АПК). При этом суперантиген, минуя этот необходимый для специфического распознавания этап, способен одновременно связывать молекулы MHC II класса на поверхности АПК и фрагмент вариабельной части β-цепи Т-клеточного распознающего рецептора на поверхности Т-клетки, имитируя таким образом узнавание антигена Т-клеточным рецептором. В результате происходит активация поликлональных Т-лимфоцитов с массивным высвобождением цитокинов, участвующих в воспалительных процессах, связанных с различными заболеваниями, такими как РА, СД1, РС, СКВ и др.
Поэтому в последние десятилетия изучение роли инфекционных (вирусно-бактериальные ассоциации) и наследственных факторов в развитии хронических воспалительных заболеваний суставов у детей сохраняют свою актуальность. Ювенильный артрит (ЮА) остается одной из наиболее обсуждаемых проблем современной детской ревматологии и педиатрии в целом, поскольку является распространенной патологией среди воспалительных ревматических заболеваний детского возраста [33–35].
Согласно современным представлениям, ЮА рассматривается как обобщающее понятие, объединяющее гетерогенную группу хронических заболеваний суставов, имеющих различный этиопатогенез и иммуногенетическое происхождение, различную нозологическую принадлежность и неоднозначный прогноз. В группу ЮА можно отнести ЮИА, ювенильный ревматический артрит (ЮРА) и ювенильный спондилоартрит (ЮСА). Среди ЮА наиболее значимым является ЮИА, приводящий к инвалидизации в детском возрасте [33, 35–37]. ЮИА, как и ЮА в целом, представляет гетерогенную группу, в которой выделяют разные формы и варианты течения. Традиционно принято выделение системных (суставно-висцеральных) и суставных форм [36].
По результатам исследований [34–35, 36–40], распространенность ЮА в разных странах колеблется от 0,05 до 0,6%, а заболеваемость – от 2 до 19 случаев на 100 тыс. детского населения в возрасте до 16 лет. Распространенность ЮА среди детей до 18 лет на территории Российской Федерации достигает 62,3 на 100 тыс., первичная заболеваемость – 16,2 на 100 тыс., в том числе среди подростков – соответственно 116,4 и 28,3, среди детей до 14 лет – 45,8 и 12,6 [35].
ЮА относятся к мультифакториальным заболеваниям, в развитии которых играют роль не только экзогенные (инфекционные и средовые), но и наследственные факторы, в том числе иммуногенетические [25, 34, 36, 41–43].
Отсутствие окончательного представления об этиологии и патогенезе, а также полиморфизм клинической картины ЮА создают ряд проблем в вопросах их диагностики и лечения.
Развитие и прогрессирование ЮИА определяется сочетанием генетически детерминированных и вторичных дефектов иммунорегуляторных механизмов, приводящих к быстрой трансформации физиологической острой воспалительной реакции в хроническое прогрессирующее воспаление. Однако механизмы становления аутоиммунного патогенеза ЮРА довольно сложны и, несмотря на многочисленные исследования, до конца не раскрыты [44, 45].
Таким образом, с одной стороны, большинство авторов признают ведущую роль в патогенезе ЮРА генетически детерминированного дисбаланса клеточного звена иммунной системы, в частности, CD4+- и Th1-лимфоцитов, моноцитов (макрофагов), секретируемых ими про- и противовоспалительных цитокинов. Развитие заболевания связывают с аутоантителами к IgG и возможностью его опосредования иммунными комплексами с привлечением полиморфноядерных лейкоцитов [46]. Но вопрос о соотношении и роли разных звеньев иммунокомплексного воспаления в патогенезе ЮРА остается дискуссионным. Спектр исследований, направленный на формирование научно обоснованной концепции патогенеза ЮИА за последние годы начинает смещаться в область молекулярно-биологических разработок иммуногенетического статуса заболевания: детализируются отклонения в биохимических профилях иммунной и генетической системы, определяются алгоритмы их взаимоотношений. В аспекте генетических исследований всё больше внимания уделяется повреждениям генетического аппарата отдельных клеток и механизмов, а также феномену нестабильности генома у больных ЮРА [47].
С другой стороны, ЮИА нередко сопровождается активацией коморбидной инфекции, и вирусно-бактериальное инфицирование у детей с ЮРА является прогностически неблагоприятным признаком, указывающим на бóльшую тяжесть болезни с возможностью возникновения необратимых процессов с нарушением функции суставов, развитием висцеритов и васкулитов [36, 48].
У детей с ЮИА выявляется 2 варианта инфицирования вирусами и бактериями: в начале заболевания и спустя несколько месяцев и лет от его дебюта. Во всех случаях как носительства, так и острой инфекции ЮРА протекает в виде более тяжелых клинических форм [49].
Нерешенным остается вопрос об уровне взаимосвязи инфекционных и генетических факторов в развитии и течении ЮА. По мнению ряда авторов [26, 31, 50], не исключается возможность инициации инфекционным агентом артрита, особенно у генетически предрасположенного больного. В отечественной и зарубежной литературе имеются данные о том, что ГВИ у детей с ЮИА не только утяжеляют его течение, но и могут участвовать в реализации патогенетических иммунокомплексных процессов [14, 49–51].
Имеются публикации о том, что, по меньшей мере, часть случаев РА, ювенального хронического артрита и так называемых серонегативных спондилоартропатий могут иметь вирусную этиологию [52]. Так, у некоторых больных прослеживается непосредственная связь развития хронического артрита с предшествовавшей вирусной инфекцией. Хронический синовит может быть обусловлен продолжительной местной антигенной стимуляцией вследствие персистирования вирусных агентов непосредственно в клетках синовиальной оболочки либо в результате систематического реинфицирования суставных тканей при наличии в организме устойчивого экстраартикулярного депо вирусной инфекции [53].
Фармакотерапия ЮА, в частности, ЮИА, остается одной из наиболее сложных проблем современной клинической медицины. К основным направлениям лечения ЮРА относятся стабилизация патологического процесса, предотвращение его обострения, а также реабилитация функциональных нарушений суставов, возникающих у ребенка в результате болезни. Лекарственная терапия больных ЮРА основана на применении трех групп медикаментов – НПВП, глюкортикоидов и базисных препаратов. При этом установлено, что результаты лечения зависят от формы болезни и сроков назначения терапии. Определена целесообразность сочетания симптом-модифицирующих средств (НПВП и внутрисуставные глюкокортикоиды) с метотрексатом, а при наличии инфекции – с внутривенными иммуноглобулином.
Согласно рекомендациям EULAR, иммуносупрессивную терапию следует начинать как можно раньше после установления диагноза, что позволяет не только снизить иммунное воспаление, но и затормозить деструкцию в суставах, а следовательно, сохранить трудоспособность и качество жизни пациентов. Наряду с «золотым стандартом» базисной противовоспалительной терапии метотрексатом в последние десятилетия используется биологическая терапия (антицитокиновые моноклональные антитела, антитела к рецепторам иммунокомпетентных клеток и др. Нельзя не учитывать тот факт, что использование генно-инженерных биологических препаратов, мишенью которых являются узловые патогенетические звенья воспалительного процесса (ФНО-α, ИЛ-1, ИЛ-6, В-клетки и т. д.) способствуют не только подавлению аутоиммунных реакций, но и создают условия для реактивации латентных вирусных инфекций [48, 54–57]. Это часто не только затрудняет проведение терапии базисными препаратами, лимитируя их использование, но и может определять неблагоприятные исходы основного заболевания за счет поддержания аутоиммунного процесса и формирования порочного патологического круга [58].
В то же время в современной литературе встречаются лишь единичные работы, посвященные изучению эффективности пpотивовирусных препаратов в педиатричекой ревматологии [58, 59]. Дискуссионными остаются вопросы о возможности и длительности применения этих препаратов у детей, целесообразности их использования при разных стадиях и клинических вариантах ЮА.
Поэтому вопрос о роли ГВИ как причинного агента, усугубляющего дефект иммунорегуляторных систем и обусловливающего развитие иммунных нарушений, лежащих в основе ЮИА, является актуальным. Его решение позволит не только определять факторы, способствующие развитию заболевания, но в ряде случаев обозначит перспективы прогнозирования его клинического течения.


