В декабре 2019 г. в Ухане (КНР) возникла эпидемическая вспышка острого респираторного заболевания COVID- 19, ассоциированного с вирусом SARS-CoV-2. Инфекция стремительно распространилась по всей территории Китая. 31 декабря 2019 г. ВОЗ признала вспышку инфекции, а 30 января 2020 г. объявила чрезвычайную ситуацию на международном уровне и глобальную пандемию – 11 марта 2020 г. Случаи коронавирусной инфекции были зарегистрированы в большинстве стран мира [1].
С начала пандемии эпидемическая ситуация по COVID- 19 динамично изменяется. В 2021 г. широкое распространение получил сначала вариант коронавируса дельта, характеризующийся более высокой смертностью, а затем южноафриканский вариант – омикрон, отличающийся значительной скоростью распространения в человеческой популяции за счет высокого коэффициента передачи [1].
Фавипиравир (Ф) является ингибитором РНК-зависимой РНК-полимеразы (RdRp) – ключевого белка, участвующего в репликации генома вируса SARS-CoV-2. Попадая в клетку, он метаболизируется до активного метаболита трифосфата Ф, который, связываясь с RdRp, эффективно подавляет размножение вируса двумя возможными путями: остановкой синтеза РНК после присоединения двух остатков фавипиравира в растущую цепь [2, 3], либо встраиванием в РНК вируса и индукцией летального мутагенеза [4–6]. Ф активен в отношении различных классов вирусов с РНК-геномами, в том числе из семейства Coronaviridae [7–11]. При этом основным механизмом действия в отношении SARS-CoV-2 является индукция летального мутагенеза вследствие аккумуляции ошибок в геноме вируса [11]. Ранее была продемонстрирована активность препарата в отношении SARS-CoV-2 в исследованиях in vitro [12–14] и in vivo [12, 15], а также в ряде клинических исследований [16–21], что позволило внести препараты на основе Ф в клинические рекомендации по лечению новой коронавирусной инфекции [22]. Однако большинство исследований было проведено на ранних вариантах SARS-CoV-2. В ряде более поздних клинических исследований Ф не показал статистически значимого различия по сравнению с плацебо [23–26], это дало основания предположить, что, хотя изменения в гене RdRp вследствие эволюции вируса незначительны, Ф мог утратить свою эффективность в отношении новых вариантов SARS-CoV-2 дельта и, возможно, омикрон. Таким образом, возникла необходимость оценки эффективности ингибирования Ф новых вариантов SARS-CoV-2 для понимания возможности его дальнейшего использования в лечении новой коронавирусной инфекции. Поэтому настоящее исследование было посвящено изучению фармакологической активности Ф in vitro в отношении вариантов SARS-CoV-2 дельта и омикрон в сравнении с более ранним прототипным «европейским» штаммом клада В.1.1.
Материалы и методы
Линия клеток почки зеленой мартышки Vero получена из Biologicals (ВОЗ, Швейцария). Клетки вели на среде Игла, модифицированной Дульбекко (ДМЕМ, ФНЦИРИП им. М.П. Чумакова РАН, Россия) с добавлением 5% фетальной бычьей сыворотки (FBS, Gibco) и смеси антибиотиков (стрептомицин, 0,1 мг/мл и пенициллин penicillin 100 Ед/мл) («ПанЭко», Россия).
Штаммы вируса SARS-CoV-2 [прототипный штамм ПИК35 (клад Pango B.1.1, GISAID ID EPI_ISL_428852), вариант дельта штамма 4724d (GISAID ID EPI_ISL_8799478) и вариант омикрон штамма 7995o (GISAID ID EPI_ISL_9613539)] были выделены в Москве из назофарингеальных мазков пациентов с COVID-19. Вирусы прошли 3–5 пассажей в клетках Vero cells и хранились в виде замороженной суспензии зараженных клеток при -70 °C.
Готовили серию 2-кратных разведений стоковых растворов субстанции и контрольного соединения, начиная с концентрации 1:50, на среде ДМЕМ. Для Ф диапазон концентраций составлял 62,5–1000 мкМ, для контрольного препарата N-гидроксицитидина (NHC) – 6,25–100 мкМ. Приготовленные разведения добавляли к клеткам Vero и инкубировали 4 ч при 37 °С в СО2-инкубаторе. Готовили рабочие разведения вирусов для заражения клеток с 0,0001 MOI (множественность заражения) (ТЦД50/кл) и добавляли их к клеткам с соединениями. Клетки инкубировали 2 ч при 37 °С в СО2-инкубаторе для адсорбции и проникновения вируса, затем среду с вирусом удаляли и заменяли на соответствующие разведения соединений. После чего инкубировали клетки при 37 °С в СО2-инкубаторе 40 ч для прототипного штамма ПИК35 и 4724d варианта штамма дельта, 114 ч – для 7995о варианта штамма омикрон. После инкубации количество вирусной РНК оценивали в супернатанте с помощью коммерческого набора реагентов ПОЛИВИР SARS-CoV-2 (НПФ «Литех», Россия) для полимеразной цепной реакции с обратной транскрипцией (ОТ-ПЦР) c детекцией в режиме реального времени согласно инструкции производителя, определяли Ct (пороговый цикл флуоресценции). В каждом эксперименте проводили титрование препарата позитивного (N-гидроксицитидин, активный метаболит молнупиравира) и отрицательного контроля (растворитель без носителя). Эксперимент независимо повторен 2 раза для расчета среднего значения ЕС50.
Готовили серию 2-кратных разведений стоковых растворов, начиная с 1000мкМ, на среде ДМЕМ (ФНЦИРИП им. М.П. Чумакова РАН, Россия). Приготовленные разведения добавляли к клеткам Vero и инкубировали 40 ч при 37 °С в СО2-инкубаторе. Затем среду удаляли и заменяли на 0,03 мг/мл раствор резазурина в среде ДМЕМ. Клетки инкубировали 4 ч при 37 °С в СО2-инкубаторе. Флуоресценцию детектировали при 544 nm Ex/590 nm Em (Fluoroskan, ThermoFisher Scientific, США).
Результаты и обсуждение
Использованная в исследовании клеточная линия Vero, хотя и не является клеточной линией легочного эпителия, характеризуется высоким уровнем экспрессии ангиотензинпревращающего фермента 2 (ACE2) на поверхности клеток, который служит рецептором для вируса SARS-CoV-2, отвечая за связывание и проникновение его в клетки [27, 28]. Эта клеточная линия широко применяется в рамках исследований SARS-CoV-2 и скрининга противовирусных соединений in vitro.
В более ранних исследованиях фармакологическую активность Ф in vitro изучали на клеточных культурах VeroE6 [11–14], Calu-3 [29], а также Caco-2 [12, 29]. Данные по ингибирующей активности препарата в разных исследованиях сильно разнились в зависимости от дизайна эксперимента (вида вируса, диапазона исследованных концентраций, времени инкубации с вирусом и активным веществом, MOI и др.), а также детектируемого параметра (концентрации вирусной РНК в супернатанте, цитопатического эффекта или окрашивания по белку S). Таким образом, диапазон ингибирующих концентраций (EC50) для Ф составлял от 62 до более 500 мкМ. При этом в части исследований ЕС50 не была достигнута из-за ограниченного диапазона исследуемых концентраций [14, 29, 30].
Мы подобрали оптимальные условия для проведения экспериментальной оценки противовирусной активности Ф in vitro, при которых отсутствовали как цитотоксичность, вызванная инкубацией с препаратом, так и цитопатический эффект вируса, которые могут затруднить интерпретацию полученных результатов. В данном исследовании клетки Vero инкубировали в течение 4 ч с Ф в серийных разведениях от 32,5 мкМ до 1000 мкМ, после чего к ним добавляли вирус в дозе MOI 0,0001 и инкубировали с клетками в течение 40 ч для прототипного штамма и варианта дельта и 114 ч – для варианта омикрон из-за более медленной репликации вируса и отсутствия значимых различий между положительным и отрицательным контролем после инкубации в течение 40 ч. Концентрацию вирусного урожая в супернатанте определяли методом ОТ-ПЦР с детекцией в реальном времени.
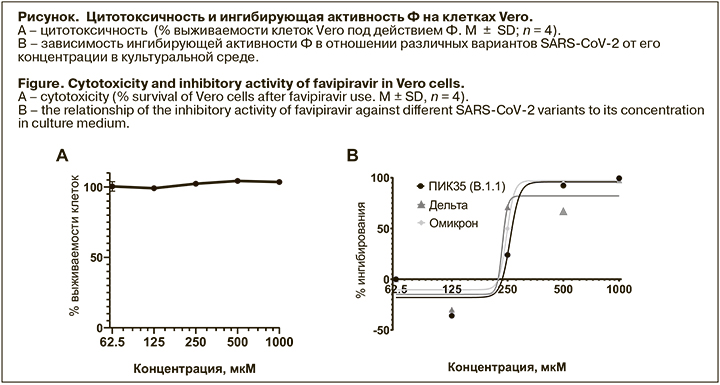
Ф ингибировал репродукцию штаммов всех трех вариантов SARS-CoV-2 в диапазоне концентраций 200 –300 мкМ (см. рисунок), при этом отсутствовали статистически достоверные различия в значениях ингибирующих концентраций ЕС50 между штаммами (см. таблицу). Кроме того, не было выявлено токсического эффекта Ф в исследованном диапазоне концентраций при инкубации с клетками как в течение 40 ч, так и при более длительной экспозиции (144 ч). Таким образом, ингибирующая активность Ф сохраняется в отношении появляющихся новых вариантов SARS-CoV-2.
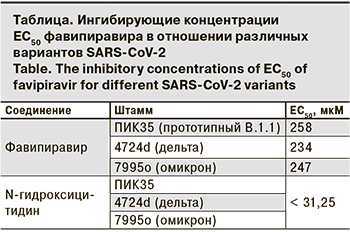
Анализ мутаций вариантов SARS-CoV-2 показал, что изменения в основном затрагивают поверхностный S-белок вириона коронавируса, что приводит к существенному снижению активности нейтрализующих антител [29], специфически связывающихся с рецептор-связывающим доменом S-белка. Однако изменения в белках, играющих ключевую роль в реализации генома вируса (RdRp, протеазы и др.), не столь существенны и в основном находятся вдали от активных центров. Вследствие этого низкомолекулярные ингибиторы соответствующих мишеней не теряют своей ингибирующей активности в отношении SARS-CoV-2 (31, 32]. Виду того, что Ф является ингибитором RdRp, снижения его ингибирующей активности в отношении новых вариантов SARS-CoV-2 не происходит.
Тем не менее, Ф ингибирует SARS-CoV-2, в отличие от вируса гриппа и других коронавирусов [4, 33], в достаточно высоких концентрациях, как минимум на порядок выше, чем у других перспективных ингибиторов, например, N-гидроксицитидина, использованного в нашем эксперименте в качестве положительного контроля, который практически полностью подавлял репродукцию SARS-CoV-2 во всем диапазоне исследованных концентраций.
Согласно ряду фармакокинетических исследований, такие концентрации в плазме вполне соответствуют наблюдаемым при применении Ф в стандартном режиме дозирования [1800 мг 2 раза в день (нагрузочная доза) и 800 мг 2 раза в день в последующие дни)] с Cthrough на 3-й день терапии около 200 мкМ [33]. Длительное и стабильное поддержание уровня Cthrough в плазме выше ЕС90, являющееся одним из условий, обеспечивающих стойкий противовирусный эффект препарата, достижимо не всегда и не у всей популяции пациентов, что может приводить к смазанным показателям клинической эффективности [34]. Однако снижение уровня Cthrough не всегда означает снижение эффективности Ф, концентрация активного метаболита – трифосфата Ф – в тканях может превышать ингибирующую концентрацию в отношении РНК полимеразы SARS-CoV-2 [35, 36]. Моделирование фармакокинетики вместе с уровнями концентрации активного метаболита Ф в тканях показывает, что при стандартном режиме дозирования (1600/800 мг) достаточный уровень в тканях (20 мкМ трифосфата Ф) сохраняется как минимум в течение 3 дней, а при режиме 1600/1200 мг – до 9 дней [37]. Таким образом, применение Ф в более высоких дозах потенциально могло бы улучшить показатели его эффективности при новой коронавирусной инфекции, однако в таком случае потребуется дополнительная оценка безопасности его применения.
Заключение
В исследовании 3 разных вариантов вируса SARS- CoV-2 было показано, что эволюционные изменения вируса не привели к существенным изменениям противовирусной активности Ф in vitro. Полученные ингибирующие концентрации в целом соответствуют результатам предыдущих исследований. Таким образом, Ф остается эффективным противовирусным средством, в том числе в отношении новых вариантов SARS-CoV-2.


