В последнее время темпы развития эпидемии ВИЧ-инфекции в мире замедлились. По оценкам ЮНЭЙДС, число новых случаев заражения ВИЧ среди взрослого населения сократилось на 40% по сравнению с 1998 г., когда этот показатель достиг максимального значения и составлял 2,8 млн чел. В 2019 г. число новых случаев заражения составило 1,7 млн [1].
Однако в то время как в мире темпы развития эпидемии снижаются, в странах бывшего Советского Союза растет распространенность ВИЧ, и число новых случаев инфекции в период с 2010 по 2019 г. увеличилось на 72%. В СССР первый случай заражения ВИЧ был зарегистрирован в 1980-х годах. Ухудшение социально-экономической ситуации и свободное безвизовое перемещение привели к массовым миграциям внутри стран Содружества Независимых Государств (СНГ). Трансграничная миграция способствовала распространению эпидемии в других странах, и в результате происходящих событий в 1988 г. был зарегистрирован первый случай заражения ВИЧ в Республике Армения [2].
К концу 2019 г. оценочное число лиц, живущих с ВИЧ (ЛЖВ), в Республике Армения составляло 3500 чел., а в Российской Федерации – 1 106 513 чел. Миграционные потоки являются важным фактором передачи инфекции: из всех ВИЧ-инфицированных в стране около 57% составляют трудовые мигранты и 13% – их половые партнеры. Таким образом, 70% случаев заражения ВИЧ в стране связаны с фактором трудовой миграции. Более 90% случаев инфицирования граждан Республики Армения, которые произошли за ее пределами, приходятся на Россию, 5% – на Украину, 0,8% – на Польшу и 0,7% – на Казахстан [3, 4]. В связи с этим эпидемии ВИЧ-инфекции в Республике Армения и Российской Федерации связаны и в значительной степени влияют друг на друга.
Основные проблемы, которые препятствуют прекращению эпидемии ВИЧ/СПИДа, – высокая распространенность ВИЧ-инфекции в связи с миграционными потоками, небрежное отношение людей к собственному здоровью, низкая приверженность антиретровирусной терапии (АРТ), отсутствие возможности тестирования на лекарственную устойчивость (ЛУ) ВИЧ в рутинном порядке.
Республика Армения в составе стран ВЕЦА (Восточная Европа и Центральная Азия) присоединилась к стратегии ЮНЭЙДС «90–90–90» для решения проблем развития резистентности к антиретровирусным препаратам (АРВП) и достижению прекращения эпидемии ВИЧ/СПИДа как угрозы общественному здравоохранению к 2030 г. Цель «90–90–90» гласит, что 90% ЛЖВ должны знать свой ВИЧ-статус, 90% людей с диагнозом ВИЧ – получать АРТ и 90% из них должны иметь неопределяемый уровень вируса в крови [5]. В 2019 г. число людей, знающих о своем ВИЧ-статусе, в Армении составило 77%, в России – 73%. Большой разрыв наблюдался между тестированием на ВИЧ и предоставляемым лечением: в России АРТ получали 50% ЛЖВ, а Армении доступ к лечению имели 63% больных ВИЧ-инфекцией. В результате в России всего у 38% ЛЖВ наблюдалось подавление вирусной нагрузки (ВН), в Армении этот показатель был выше и составил 55% [3].
АРТ широко применяется в Республике Армения, в том числе в рамках глобальной стратегии ВОЗ по борьбе с распространением ЛУ ВИЧ. По состоянию на 2017 г. АРВП в Армении предоставляются всем взрослым пациентам с ВИЧ, независимо от клинической стадии и количества CD4+-лимфоцитов. На сегодняшний день разработано обновленное клиническое руководство по профилактике и лечению ВИЧ- инфекции, основанное на рекомендациях ВОЗ 2016 г. [6]. В Армении применяются схемы терапии первого ряда на основе EFV. В качестве предпочтительных схем используют TDF + ЗTC (или FTC) + EFV; TDF + ЗTC (или FTC) + DTG. Альтернативными являются AZT + ЗTC + EFV (или NFP); TDF + ЗTC (или FTC) + EFV; TDF + ЗTC (или FTC) + NVP, при особых обстоятельствах в регионе назначают ABC или терапию , включающую ИП.
Современная АРТ существенно продлевает жизнь ЛЖВ и снижает риск передачи ВИЧ. Однако существующая высокоактивная АРТ может оказаться неэффективной в связи с выработкой вирусом мутаций ЛУ к АРВП. В России только 1% ЛЖВ проходят тестирование на определение ЛУ ВИЧ к АРВП, а Армения не имеет возможности проведения тестов на генотипирование ВИЧ, поэтому в настоящий момент в научной литературе данные о резистентности ВИЧ к АРВП в Республике Армения скудны. Известно, что в 2009–2010 гг. распространенность ЛУ среди наивных пациентов составила 1,5% [7].
С увеличением числа пациентов, принимающих АРТ, возрастает вероятность передачи резистентных форм вируса, поэтому увеличение охвата терапией влечет за собой необходимость исследования ЛУ ВИЧ-1 как у пациентов, находящихся на АРТ, так и у пациентов без опыта приема АРВП. Данные, полученные в результате анализа, необходимы, в том числе, для эпидемиологического надзора за резистентностью, так как увеличение количества используемых препаратов может ухудшить эффективность терапевтических мероприятий в стране.
В связи с этим целью исследования являлось изучение особенностей передачи лекарственно устойчивых вариантов ВИЧ-1 в Республике Армения и степень их генетического родства с вирусами, циркулирующими в Российской Федерации.
Материалы и методы
В период с 2017 по 2019 г. была собрана коллекция образцов плазмы крови (n = 554) от граждан Республики Армения. Пациентов включали в исследование последовательно, во время рутинных визитов в Республиканский центр по профилактике СПИДа Министерства здравоохранения Республики Армения. В исследование были включены пациенты с диагнозом «ВИЧ-инфекция», достигшие 18 лет на момент проведения исследования; пациенты, находившиеся под диспансерным наблюдением, а также проходившие обследование и сдавшие кровь до начала данного исследования. От каждого пациента было получено информированное согласие на участие в исследовании. Все стадии исследования соответствуют международным этическим нормам и нормативным документам исследовательских организаций, а также одобрены локальным этическим комитетом Центрального НИИ эпидемиологии Роспотребнадзора (далее – ЦНИИЭ).
На момент забора крови у всех пациентов не было опыта приема АРТ.
Средний возраст пациентов составил 43 (18–72) года. Среди пациентов, включенных в исследование, 68,% составляли мужчины, 31,9% – женщины. Среди обследованных с известным путем заражения преобладал половой путь – 90% (гетеросексуальные контакты – 81,0%, гомосексуальные – 9,0%), парентеральный (прием инъекционных наркотиков) – 9,3%. У 0,7% путь заражения ВИЧ был неизвестен. Даты положительного иммунного блота варьировали с 2014 по 2019 г.
Демографические, клинические и эпидемиологические данные участников были получены из их медицинских карт.
Образцы крови исследовали путем массового параллельного секвенирования с помощью набора «АмплиСенс HIV-Resist-NGS» (ЦНИИЭ, Россия). Выделение РНК из плазмы крови, амплификацию и секвенирование региона pol (позиции 2253–3368 пн референсного штамма HXB-2, регистрационный номер в GenBank К03455), кодирующего протеазу и часть обратной транскриптазы ВИЧ, проводили на приборе MiSeq (Illimina, США).
Анализ образцов плазмы крови путем классического секвенирования по методу Сенгера осуществляли с помощью набора реагентов «АмплиСенс HIV-Resist-Seq» (ЦНИИЭ, Россия). Секвенирование очищенных фрагментов проводили, используя генетический анализатор Applied Biosystems (ThermoFisher Scientific, США) в соответствии с инструкциями производителя. Обработку данных секвенирования и получение консенсусной нуклеотидной последовательности осуществляли с помощью программного обеспечения «ДЕОНА» (v. 1.7.0) («РМбит», Россия).
Для оценки качества нуклеотидных последовательностей ВИЧ-1 использовали инструмент WHO HIV DR (http://pssm.cfenet.ubc.ca/who_qc/) перед анализом данных.
Дальнейшую работу с нуклеотидными последовательностями (множественное выравнивание, расчет генетических дистанций и построение филогенетических деревьев) проводили с помощью программного обеспечения BioEdit 7.2.0 и MEGA (v. 6.0) с использованием статистического метода Maximum Likelihood analysis (bootstrap level 100). Субтипы ВИЧ-1 были определены с использованием базы данных Стэнфордского университета и впоследствии подтверждены филогенетическим анализом. Для образцов, давших сомнительный результат генотипирования и/или филогенетического анализа, проводили дополнительный анализ в приложении HIV BLAST сайта международной базы данных института Los Alamos (https://www.hiv.lanl.gov/content/index). Молекулярные кластеры выявляли с помощью программного обеспечения Cluster Picker 1.2.3 с максимальной генетической дистанцией 0.045 нуклеотидных замен на позицию с bootstrap поддержкой более 90%.
Мутации ЛУ выявляли с помощью базы данных Стэнфордского университета HIVdb (v. 8.5) (https://hivdb.stanford.edu/) [8]. Помимо выявленных мутаций резистентности был определен уровень ЛУ. Оценку мутаций резистентности из листа SDRM 2009 г. для надзора за передаваемой ЛУ проводили с помощью инструмента CPR (v. 6.0) (https://hivdb.stanford.edu/cpr/) [9].
Результаты
Характеристика исследуемой популяции
Проанализировано 546 нуклеотидных последовательностей, прошедших контроль качества ВОЗ. Результаты генотипирования и последующий филогенетический анализ позволили установить, что доминирующим субтипом в Республике Армения является А1 (согласно новой классификации, суб-субтип А6) – 447 (87,4%) случаев [7, 10]. У 32 (5,9%) пациентов был выявлен субтип В. В 35 (6,4%) образцах обнаружены циркулирующие рекомбинантные формы: CRF02_AG – у 13 (2,4%) чел., CRF63_02A1 – у 8 (1,5%), CRF24_BG – у 5 (0.9%), CRF06_AB – у 4 (0,7%), CRF06_cpx – у 4 (0,7%), CRF63_02A – у 1 (0,2%). У 2 (0,4%) чел. был обнаружен субтип С, который отвечает за большинство случаев заражения по всему миру [11].
Оценка распространенности мутаций резистентности и прогнозирование ЛУ
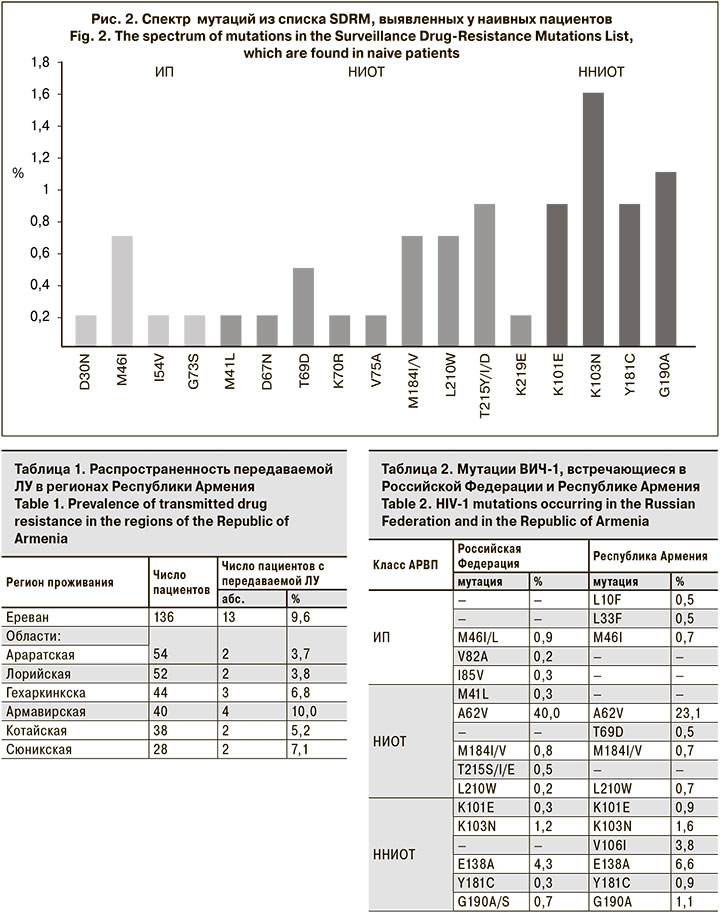
Были проанализированы частота возникновения и структура мутаций ЛУ к ингибиторам протеазы (ИП), нуклеозидным ингибиторам обратной транскриптазы (НИОТ), ненуклеозидным ингибиторам обратной транскриптазы (ННИОТ), а также прогнозируемая ЛУ к АРВП, ассоциированная с обнаруженными мутациями. В результате хотя бы 1 мутация резистентности была выявлена у 89 (16,3%) пациентов. Однако только у 30 (5,5%) наивных пациентов были найдены надзорные мутации ЛУ, согласно списку SDRM 2009 г. [9]. Чаще всего их выявляли к ННИОТ – у 24 (4,4%) пациентов; реже встречались мутации, ассоциированные с ЛУ к НИОТ – у 11 (2,0%) пациентов; только у 1 (0,2%) пациента была обнаружена ЛУ к ИП (рис. 1).
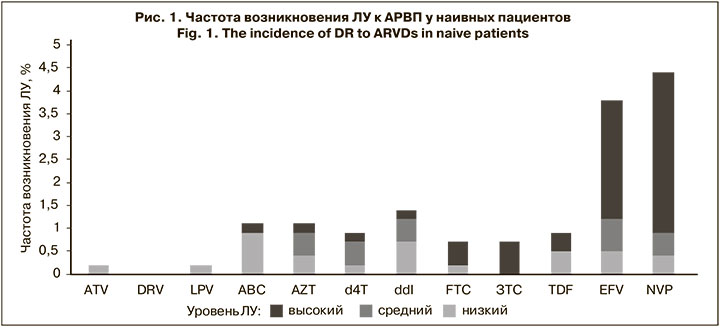
Анализ распространенности мутаций, ассоциированных с ЛУ низкого, среднего и высокого уровней к АРВП, показал, что наиболее часто они развивались к препаратам класса ННИОТ. В большинстве случаев была обнаружена полиморфная мутация E138A, распространенная у суб-субтипа А6 и ассоциированная с ЛУ низкого уровня – у 36 (6,6%) чел. Кроме того, были выявлены мутации, вызывающие низкий уровень ЛУ: V106I – у 21 (3,8%) чел., V179D/E/T/L – у 10 (1,9%), V108I – у 6 (1,1%), K101E – у 5 (0,9%), A98G – у 3 ( 0,5%). У 5 (0,9%) пациентов была определена мутация Y181C, вызывающая средний уровень устойчивости к EFV и высокий – к NVP. Были обнаружены мутации K103N и G190A, ассоциированные с ЛУ высокого уровня к EFV и NVP – у 9 (1,6%) и 6 (1,1%) чел. соответственно. Схожий профиль мутаций у пациентов с выявленной ЛУ к EFV и NVP связан с перекрестной резистентностью этих препаратов.
Меньшая частота распространенности мутаций была обнаружена к НИОТ. Полиморфная мутация A62V, свойственная суб-субтипу А6, распространенная в странах бывшего СССР и не вызывающая ЛУ, встречалась наиболее часто – у 126 (23,1%) пациентов. У 7 (1,2%) чел. были выявлены мутации M184I, L210W, T215D, ассоциированные с ЛУ низкого уровня, одновременно к 3 препаратам – ABC, ddi, TDF. У 4 (0,7%) и 5 (0,9%) чел. были выявлены мутации L210W и T215Y/I/D соответственно, которые являлись причиной возникновения ЛУ к AZT и d4T. Мутация M184V/I, обнаруженная у 4 (0,7%) чел., вызывала высокий уровень устойчивости к FTC и ЗTC. Мутация T69D, ассоциированная с ЛУ среднего уровня к ddI, была выявлена у 3 (0,5%) чел. У 1 пациента был установлен высокий уровень ЛУ ко всем НИОТ, нуклеотидная последовательность вируса содержала мутации E40F, M41L, D67N, K70R, V75A, M184V, L210W, T215Y, K219E.
Еще реже мутации, ассоциированные с ЛУ, возникали к ИП. У 4 (0.7%) человек выявлена мутация M46I, у 3 (0,5%) – L10F и L33F, которые вызывали низкий уровень ЛУ к препаратам этого класса. По 1 (0,2%) пациенту имели надзорные мутации D30N, G73S и I54V), при этом последняя вызывала низкий уровень устойчивости к ATV и LPV.
Спектр надзорных мутаций из списка SDRM, выявленных у наивных пациентов, представлен на рис. 2.
Была проанализирована зависимость между распространенностью надзорных мутаций ЛУ и характеристиками пациентов (пол, путь заражения, город/регион проживания, год положительного иммунного блота) и вирусов (субтип).
Надзорные мутации ЛУ у мужчин [4,8% (95% ДИ 16,9–38,0)] встречались значительно чаще, чем у женщин [0,4% (95% ДИ 0,2–7,2)]. Кроме того, у пациентов с субсубтипом А6 [2,7% (95% ДИ 8,4–24,7)] частота возникновения мутаций из списка SDRM была в 2 раза выше, чем у пациентов с субтипом В [1,1% (95% ДИ 2,2–13,0)]. Отличия, полученные для других субтипов, были недостоверны в связи с недостаточным объемом выборки.
У пациентов с предполагаемым гетеросексуальным путем заражения [3,5% (95% ДИ 11,4–29,7)] надзорные мутации встречались в 3 раза чаще, чем при гомосексуальном пути передачи ВИЧ [1,3% (95% ДИ 2,8–14,4)]. Вероятно, эта зависимость объясняется тем, что за последние годы примерно 60% новых случаев заражения происходят при гетеросексуальных контактах. Но достоверной зависимости между распространением передаваемой ЛУ и путем заражения выявлено не было.
У пациентов, проживающих в Ширакской (n = 50), Агарацотнской (n = 18), Тавушской (n = 17) и Вайодзорской (n = 11) областях надзорные мутации из списка SDRM не обнаружены. Эти регионы были исключены из анализа. Наиболее высокий уровень распространенности передаваемой ЛУ был у пациентов из Армавирской области (10,0%) и Еревана (9,6%), что объясняется, вероятно, более длительным применением АРВП на данных территориях по сравнению с другими регионами (табл. 1).
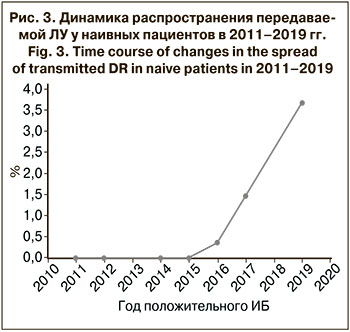
В динамике распространения ЛУ среди пациентов без опыта приема АРТ в период с 2011 по 2019 г. наблюдается тенденция к увеличению уровня резистентности ВИЧ-1 (рис. 3). Это связано с началом широкомасштабного применения АРВП в Республике Армения с 2017 г. [6].
Для анализа взаимосвязи эпидемий ВИЧ-инфекции в Республике Армения и Российской Федерации мы сравнили нуклеотидные последовательности циркулирующих вариантов вирусов. Было установлено, что в России доминирует суб-субтип А6 (82,8%), распространен также субтип В (8,2%). Эти же подтипы преобладают и в Армении (87,4 и 5,9% соответственно). Кроме того, в обеих странах встречаются общие циркулирующие рекомбинантные формы, в частности, CRF63_02A1 (4,2 и 1,5% соответственно) и CRF02_AG (0,5 и 2,4%) [12].
Как в Армении, так и в России наибольшее число мутаций, ассоциированных с ЛУ, было выявлено к ННИОТ [13], реже – к НИОТ и ИП. В обеих станах распространены полиморфные мутации E138A и А62V. Также часто встречаются мутации K103N и G190A/S, которые приводят к развитию резистентности высокого уровня к EFV и NVP. Относительно НИОТ преобладает мутация M184I/V, вызывающая высокий уровень устойчивости к FTC и ЗTC. И только одна мутация M46I/L из группы ИП распространена в обеих странах. Перечень наиболее распространенных мутаций, встречающихся в России и Армении, представлен в табл. 2.
Анализ молекулярных кластеров
Для определения особенностей распространения мутаций ЛУ мы провели анализ молекулярных кластеров. Были изучены 554 нуклеотидные последовательности от ЛЖВ, проживающих в Республике Армения, а в качестве группы сравнения использовали 833 нуклеотидные последовательности от ВИЧ-инфицированных граждан Российской Федерации, не имевших опыта приема АРВП. Филогенетический анализ 1387 нуклеотидных последовательностей определил 135 молекулярных кластеров, содержащих 341 последовательность, что составило 24,59% от всех использованных для анализа сиквенсов. При этом доля кластеризовавшихся последовательностей от ЛЖВ из Республики Армения была значительно выше, чем от ВИЧ-инфицированных граждан Российской Федерации – 170 (30,69%) и 172 (20,65%) соответственно. С большой долей вероятности это связано с бóльшей плотностью исследуемой выборки в Республике Армения. Среди выявленных кластеров превалировали небольшие, содержащие 2 или 3 последовательности (118 из 135). Важно отметить, что в основном кластеры образовывались нуклеотидными последовательностями из одной страны. Смешанных кластеров, которые включали сиквенсы ВИЧ-инфицированных обеих стран одновременно, было всего 3, и состояли они суммарно из 7 нуклеотидных последовательностей, что составило 2,05% от всех кластеризовавшихся сиквенсов.
Далее кластерный анализ проводили только для нуклеотидных последовательностей из Республики Армения. Сиквенсы, содержащие мутации ЛУ, кластеризовались реже, однако различие было недостоверно (17,06% против 20,05%; р = 0.409). Мы исключили из анализа мутацию A62V как высокополиморфную.
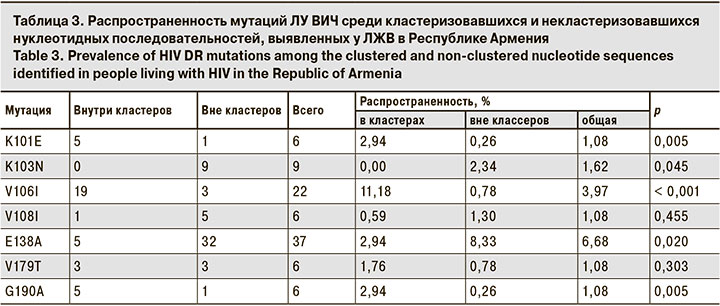
Анализ особенностей кластеризации последовательностей с мутациями, частота встречаемости которых в исследуемой выборке была больше 1%, показал, что мутации K101E, V106I и G190A достоверно чаще встречались в кластеризовавшихся последовательностях, а мутации K103N и E138A – в последовательностях вне кластеров (табл. 3).
Заключение
В результате анализа 546 нуклеотидных последовательностей фрагмента гена pol, кодирующего протеазу и часть обратной транскриптазы, от пациентов без опыта приема терапии было установлено, что уровень распространенности мутаций, ассоциированных с ЛУ, составил 16,3%. Уровень передаваемой ЛУ в результате оценки мутаций из списка SDRM 2009 г. составил 5,5%, что по классификации ВОЗ считается умеренным [14, 15]. Следует отметить значительный рост ЛУ к АРВП по сравнению с данными 2009–2010 гг., когда показатель составлял 1,5% [7].
Наиболее высокий уровень устойчивости был обнаружен к ННИОТ EFV и NVP. Часто встречались ассоциированные с ЛУ к этим препаратам мутации K103N и G190A. Среди НИОТ высокая устойчивость была обнаружена к FTC и 3ТС. M184I/V приводила к снижению восприимчивости к этим препаратам. Можно сделать вывод, что препараты, применяемые в настоящий момент в Армении в схемах АРТ первого ряда, эффективны, за исключением EFV, к которому у 3,8% наивных пациентов развивается высокий уровень устойчивости.
Анализ генетических вариантов ВИЧ-1 в Республике Армения и Российской Федерации показал, что развитие эпидемии протекает аналогично. В обеих странах в значительной степени доминируют мутации естественного полиморфизма A62V и E138A, характерные для суб-субтипа А6. Были выявлены также субтип В и 2 циркулирующие рекомбинантные формы CRF63_02A1 и CRF02_AG. В России у наивных пациентов чаще выявляли мутации, ассоциированные с ЛУ высокого уровня к препаратам классов ННИОТ (NVP и EFV) и НИОТ (FTC и 3ТС) [13]. Все эти показатели свидетельствуют о большой генетической схожести эпидемий, протекающих в разных странах.
Несмотря на высокую связанность эпидемий в двух странах и их схожесть в отношении профиля циркулирующих вариантов вируса и выявляемых мутаций ЛУ, смешанных молекулярных кластеров обнаружено очень мало (2,05%). Возможно, это связано с тем, что плотность выборки ВИЧ-инфицированных из Российской Федерации крайне мала. В нашей работе не было выявлено связи между ЛУ и степенью кластеризации нуклеотидных последовательностей в целом. Однако ряд мутаций ЛУ ВИЧ выявляли внутри кластеров достоверно чаще (K101E, V106I, G190A), а некоторые – достоверно реже (K103N, E138A).
Поскольку, согласно рекомендациям ВОЗ, Республика Армения с 2017 г. назначает терапию всем взрослым пациентам, независимо от количества CD4+-лимфоцитов и клинической стадии заболевания, охват больных АРТ за последние годы увеличился. Поэтому необходимо проводить рутинный мониторинг резистентности в регионе для контроля и предотвращения роста ЛУ к АРВП, своевременной смены назначаемых схем терапии и предотвращения передачи лекарственно устойчивых вариантов вируса.


