Коронавирусы человека (CoV), впервые идентифицированные в 1965 г., долгое время считались одними из многочисленныx представителей семейства возбудителей острых респираторных вирусных инфекций (ОРВИ), вызывавших легкие самоограничивающиеся случаи простудных заболеваний [1]. Средняя продолжительность недомогания составляла 5–7 дней и в большинстве случаев заканчивалась без какого-либо лечения. В 2011 г. группа норвежских исследователей на основании 9-летних наблюдений показала, что CoV вызывали заболевания нижних дыхательных путей, которые отмечались реже и проходили быстрее, чем случаи, причиной которых был респираторно-синцитиальный вирус [2]. Первый сигнал, показавший, что CoV отнюдь не безопасные вирусы, стала вспышка атипичной пневмонии (severe acute respiratory syndrome – SARS) в г. Гуандун (КНР). Принадлежность нового возбудителя к группе β-CoV 15 была впервые установлена марта 2003 г. [3, 4]. Позднее вспышка распространилась на 29 стран Европы, Северной Америки и Азии [5]. Всего в в отмеченный период SARS заболели более 8000 человек, из которых 774 умерли. Вспышка завершилась 5 июля 2003 г., когда ВОЗ объявила о разрыве цепи передачи SARS-CoV на Тайване [4].
Начало второго десятилетия XXI века ознаменовалось новым пришествием патогенного CoV в 2012 г., когда в Саудовской Аравии от больного SARS-подобной пневмонией был выделен новый тип β-CoV, названный Ближневосточным респираторным синдромом (MERS-CoV) [6, 7]. Первичного хозяина точно идентифицировать так и не удалось, но при этом установлено, что промежуточным хозяином были верблюды-дромадеры [8]. Одновременно было показано, что MERS-CoV обладает эпидемическим потенциалом и способен передаваться от человека к человеку. В отличие от вспышки SARS-CoV, которая завершилась довольно быстро, заболеваемость MERS-CoV наблюдали практически до 2019 г., она охватила 23 страны мира. Всего было зафиксировано более 2500 заболевших, из которых около 900 умерли. [9].
Начало третьего этапа эволюции CoV пришлось на декабрь 2019 г., когда в г. Ухань (КНР) был зарегистрирован кластер больных необычной пневмонией [10]. Почти сразу же возбудитель был идентифицирован как новый представитель β-COV. Заболевание начало стремительно распространяться, и спустя считанные недели случаи новой инфекции регистрировали по всему Китаю и в ряде стран Западной Европы. Столь высокая контагиозность нового возбудителя побудила ВОЗ уже 11 февраля объявить мировую пандемию новой болезни, названной «coronavirus diseases-2019» (COVID-19) а вирусу было присвоено название SARS-CoV-2 [11, 12]. В конце весны 2020 г. вирус распространился практически по всему миру. Отсутствуют сведения о заболеваемости только из Северной Кореи и Туркменистана, что вероятно, обусловлено изоляционистскими системами в этих государствах, а не царящим в них эпидемическим благополучием. В летние месяцы, по сообщениям СМИ, эпидемическая ситуация несколько улучшилась, однако с приходом осени во многих регионах мира наблюдается рост заболеваемости COVID. По состоянию на 21 октября 2020 г. (период написания статьи) в мире зарегистрировано 41 192 534 случая заражения SARS-CoV-2, из них 30 621366 (74,3%) выздоровевших и 1 134 933 (2,8%) умерших. По данным ресурса, наибольшее число инфицированных выявлено в США, Индии и Бразилии – 8 526 027, 7 670 537 и 5 274 817 соответственно. Россия традиционно находилась на 4-м месте с 1 447 335 зарегистрированных заражений. На территории РФ наибольшее число верифицированных случаев приходится на Москву, Московскую область и Санкт-Петербург – 377 017, 81 843 и 53 971 соответственно.
Владимирская область среди регионов РФ занимает 53-е место по числу больных (8675 чел.), свидетельствующее об относительно невысокой интенсивности эпидемического процесса. Первый случай заболевания COVID-19 выявлен 18.03.2020 (12-я нед. 2020 г.) у водителя, прибывшего из Москвы и обратившегося в поликлинику г. Коврова. В последующие 2 нед. случаев CoV отмечено не было (см. рисунок). На 14-й нед. заболеваемость составила 0,2 на 100 тыс. населения, но уже на 15-й нед. выросла в 25 раз.
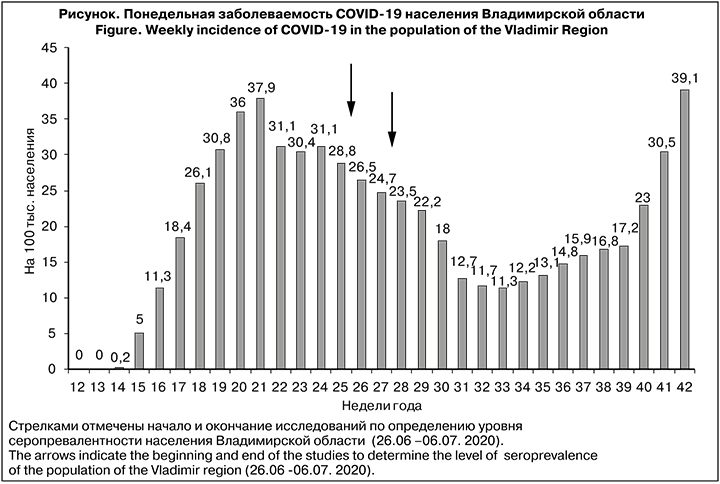
В последующие 5 нед. она стремительно нарастала с 5 до 37,9 на 100 тыс. населения.
Снижение уровня заболеваемости началось на 22-й нед. и продолжалось 12 нед., после чего начался экспоненциальный рост числа зараженных до максимального уровня, отмеченного на 42-й нед. В этой связи возникает вопрос о причинах вторичного подъема. К сожалению, убедительного объяснения пока не найдено. Можно лишь констатировать, что рост числа инфицированных SARS-CoV-2 совпал с традиционным сезонным ростом заболеваемости ОРВИ [13].
Общеизвестно, что контакт организма и любым возбудителем сопровождается формированием иммунного ответа. В этой связи SARS-CoV-2 не является исключением, и его инвазия в организм хозяина вызывает каскад клеточных и молекулярных событий, совокупность которых можно охарактеризовать как врожденный и адаптивный иммунный ответ [14]. Врожденный иммунный ответ представляет собой систему распознавания патогена и первичную реакцию, направленную на его локализацию и уничтожение. С этой целью организм использует клеточные и гуморальные факторы, такие как лимфоидно-макрофагальная система, каскад хемокинов и цитокинов и ряд других [15]. Адаптивный иммунный ответ включает короткоживущие и долговременные клетки памяти, а также систему антител, представленную IgM, IgA и IgG. [16]. Показано, что антитела IgG являются важным звеном противовирусного иммунитета, а их распределение в восприимчивой популяции (серопревалентность) в значительной степени определяет судьбу эпидемического процесса [17]. В этом смысле выбранный период определения серопревалентности населения Владимирской области в фазе снижения заболеваемости (см. рисунок) позволяет объективно оценить уровень популяционного иммунитета.
Целью исследования было определение уровня и структуры популяционного иммунитета к вирусу SARS-CoV-2 среди населения Владимирской области в период интенсивного распространения COVID-19.
Материалы и методы
Исследование проведено по единой методике оценки серопревалентности населения Российской Федерации, разработанной Роспотребнадзором при участии Санкт-Петербургского НИИЭМ им. Пастера с учетом рекомендаций ВОЗ [18]. Исследование одобрено локальным этическим комитетом НИИЭМ им. Пастера. Перед началом исследования все участники или их юридические представители были ознакомлены с целью, методикой исследования и подписали информированное согласие. Отбор волонтеров для исследования проводили методом анкетирования и последующей рандомизации.
Критерием исключения была активная инфекция COVID-19 в момент анкетирования. Объем выборки определяли по формуле:
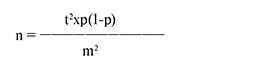
где:
n – объем выборки;
t – уровень точности (для 95% ДИ t = 1,96);
p – оценочная распространенность изучаемого явления (в данном случае при 50% = 0,5); m – допустимая ошибка – 5% [19].
Всего в анализе было использовано 2798 образцов сыворотки волонтеров. Полученные образцы при тестировании были распределены по 7 возрастным группам. Учитывая скорость созревания иммунной системы у детей, детская группа была дополнительно распределена на подгруппы: 1–6, 7–13 и 14–17 лет. Среди волонтеров были 641 мужчина и 2119 женщин, соотношение составило 23:77, то есть женщины участвовали в исследовании в 3,3 раза активнее. Доля лиц, переболевших COVID-19, верифицированных в лечебно-профилактическом учреждении, составила 2,1%, а доля волонтеров, имевших признаки ОРЗ в день обследования –2,4%.
Распределение волонтеров по районам Владимирской области было крайне неравномерным: от 1 чел. в Киржачском районе до 981 – в Коврове. Из Селивановского района данные исследований не представлены.
Пробы венозной крови в объеме 3 мл отбирали в вакутейнеры с ЭДТА и обрабатывали методом центрифугирования. Плазму отделяли от клеточных элементов, переносили в пластиковые пробирки и хранили до исследования при температуре 4 °С. Содержание антител к SARS-CoV-2 определяли методом ИФА с использованием набора реагентов для анализа сыворотки или плазмы крови человека на наличие специфических IgG к нуклеокапсиду вируса SARS-CoV-2 производства Государственного научного центра прикладной микробиологии и биотехнологии Роспотребнадзора (г. Оболенск). Результаты учитывали качественным методом и считали положительными при превышении уровня cut off.
Статистическую обработку проводили с использованием методов вариационной статистики с помощью статистического пакета Excel. Связь между уровнями заболеваемости и серопревалентности рассчитывали по программе статистического пакета Excell. Для оценки достоверности различий сравниваемых показателей использовали уровень вероятности p < 0,05.
 Результаты
Результаты
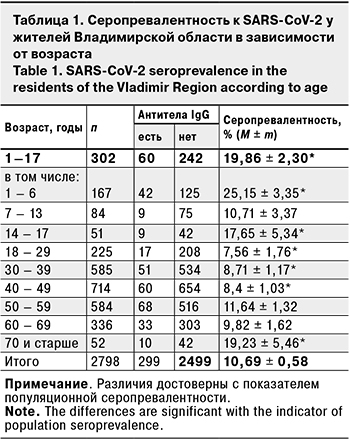
Уровень серопревалентности среди жителей Владимирской области в целом составил 10,69 ± 0.58% (табл. 1). Наибольшая доля серопозитивности отмечена в возрастных группах 1–17 лет (19,86 ± 2,30%) и 70 лет и старше (19,23 ± 5,46%). Наибольшая доля серопозитивных среди детей выявлена в подгруппе 1–6 лет (25,15 ± 3,35%), несколько меньшая – в подгруппе 14–17 лет (17,65 ± 5,34%). Интересно отметить, что в следующих трех возрастных группах – 18–29, 30–39 и 40–49 лет, составляющих наиболее активное население в физическом и социальном отношении, отмечены наименьшие показатели серопревалентности – 7,56 ± 1,76, 8,71 ± 1,17 и 8,4 ± 1,03% соответственно.
Серопревалентность не имела гендерных различий и составила у мужчин 9,05 ± 1,13%, у женщин – 10,86 ± 0,68%.
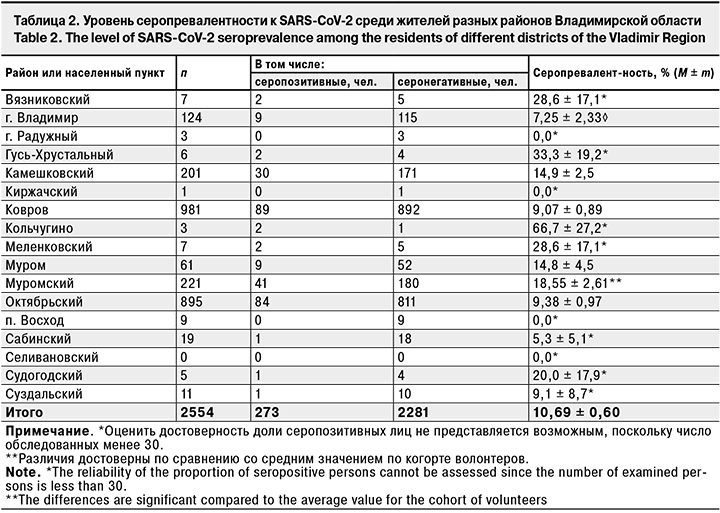
В процессе исследования коллективного иммунитета обследованы волонтеры 17 населенных пунктов. К сожалению, это обследование было крайне неравномерным (табл. 2).
Репрезентитивнная выборка была сформирована только на 5 территориях (города Владимир, Ковров и Муром, Камешковский, Муромский и Октябрьский районы). В остальных районах и населенных пунктах области выборка было нерепрезентативна. Естественно, что судить о состоянии серопревалентности на этих территориях либо невозможно совсем, либо получаемые данные имеют сугубо ориентировочное значение. Среди территорий с репрезентативными выборками наибольшая доля лиц, имевших гуморальный иммунитет к SARS-CoV-2, выявлена в Муромском районе (18,55 ± 2,61%), наименьшая – во Владимире (7,25 ± 2,33%). В то же время уровень заболеваемости в период исследования составил в Муромском районе около 2,5 на 100 тыс. населения, а во Владимире – 3,5 на 100 тыс. населения. Таким образом, отношение заболеваемости к серопревалентности в Муромском районе составило 7,42, а во Владимире – 2,07. В какой-то мере этот факт может быть объяснен разным уровнем репрезентативности: в Муромской районе было обследовано 1,4% населения, а во Владимире – только 0,03%. Разумеется, говорить о пропорциональности выборки при таком соотношении, к сожалению, не приходится, и определять какую-либо зависимость между заболеваемостью и серопревалентностью будет некорректно.
При сравнительно низкой заболеваемости не было особых оснований ожидать, что число переболевших в когорте волонтеров окажется большим. Действительно, доля их не превысила 2,1% (35 чел.). Уровень серопревалентности среди реконвалесцентов составил 57,14 ± 8,37%, тогда как среди волонтеров, отрицавших наличие COVID-18 в анамнезе, он был в 5,8 раза ниже и составил 9,83 ± 0,56%, что вполне соответствует данным по популяции в целом (см. табл. 1).
Несмотря на сравнительно низкую заболеваемость COVID-19 и малое число реконвалесцентов, около 16% волонтеров имели контакт с больными или носителями. Доля серопревалентных среди них составила 15,0 ± 1,69%, тогда как среди волонтеров, не имевших подобного контакта, – только 9,84 ± 0,62%, то есть уровень иммунитета среди контактных лиц, превышал средний по популяции в среднем в 1,5 раза (p < 0,05).
К моменту взятия проб крови для исследование на содержание специфических антител у 67 (2,4%) волонтеров были выявлены признаки ОРЗ. При этом сывороточные антитела IgG к SARS-CoV-2 выявлены у 32,83 ± 5,73% обследованных, тогда как среди здоровых этот показатель был в 3,2 раза ниже – 10,12 ± 0,57%. Поскольку ПЦР у этих лиц не проводили, можно лишь предполагать, что, по крайней мере, у некоторых из них могла быть легкая или умеренная форма COVID- 19, протекавшая под маской типичной ОРВИ.
 Отдельный интерес представили 26 человек, у которых методом ПЦР была выявлена РНК к SARS-CoV-2, но при этом отсутствовали какие-либо иные симптомы. Среди них у 42,3 ± 9,68%, в сыворотке крови были найдены специфические антитела не раньше 2-й нед. заболевания [20], поэтому можно полагать, что часть из них уже переболели в бессимптомной или легкой форме COVID-19. В этом смысле их вполне можно отнести к группе серопозитивных лиц с бессимптомным течением COVID-19.
Отдельный интерес представили 26 человек, у которых методом ПЦР была выявлена РНК к SARS-CoV-2, но при этом отсутствовали какие-либо иные симптомы. Среди них у 42,3 ± 9,68%, в сыворотке крови были найдены специфические антитела не раньше 2-й нед. заболевания [20], поэтому можно полагать, что часть из них уже переболели в бессимптомной или легкой форме COVID-19. В этом смысле их вполне можно отнести к группе серопозитивных лиц с бессимптомным течением COVID-19.
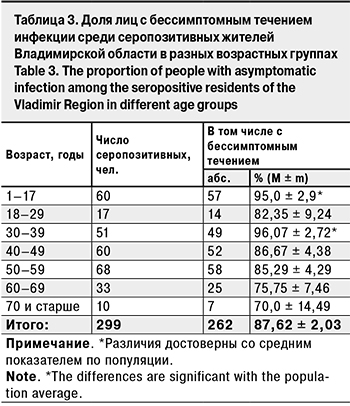
Для расчета доли бессимптомных форм COVID-19. среди серопозитивных вычисляли долю лиц, у которых отсутствует хотя бы 1 признак: диагноз COVID-19, положительная ПЦР или признаки ОРЗ. Всего было выявлено 299 серопозитивных добровольцев, среди них 87,62 ± 2,03% не имели каких-либо проявлений заболевания и были отнесены к группе лиц с бессимптомным течением (табл. 3).
Максимальное число лиц с бессимптомным течением было выявлено в возрастных группах 30–39 лет (96,07 ± 2,72%) и 1–17 лет (95,0 ± 2,9%), минимальное – среди лиц в возрасте 70 лет и старше (70,0 ± 14,49%). Результат последних следует рассматривать только как ориентировочный из-за малого объема выборки. Таким образом, полученные результаты подтверждают данные о том, что основную долю серопревалентных волонтеров составляют лица с бессимптомным течением COV-19 [18]. При этом возникает неизбежный вопрос: какова роль этой части населения в эпидемическом процессе при COVID-19?
Обсуждение
Исследование, проведенное в период с 26.06 по 06.07.2020 г., показало, что уровень серопревалентности среди населения составил всего около 10,7%, что примерно 6 раз ниже порогового уровня популяционного иммунитета (herd immunity) [21]. Это могло послужить предпосылкой повторного роста уровня заболеваемости населения, наблюдавшегося во Владимирской области с 34-й по 43-ю нед. года (срок наблюдения), причем рост числа заболевших на 43-й неделе не только не закончился, но имеет риск к переходу в экспоненциальный рост.
Что касается возрастного распределения серопревалентности, то традиционно самые высокие показатели были зарегистрированы среди детей 1–17 лет (практически 20%) и особенно в младшей возрастной группе 1–6 лет (25,1%). Максимальная доля серопозитивных среди детей отмечена и в предыдущих публикациях [18]. Неизбежно возникает вопрос относительно повышенного уровня серопревалентности среди детей. Убедительного объяснения на сегодня еще не существует. Есть только предположение, что уровень входного рецептора ACE-2, с помощью которого вирус инфицирует восприимчивые клетки, у детей наименьший , и его плотность увеличивается с возрастом [22]. Это может объяснить меньшую восприимчивость в SARS-CoV-2, но не отвечает на вопрос о повышенном уровне серопревалентности. В этом связи высказано предположение о том, что SARS-CoV-2 является новым вирусом для детского организма, и долговременные антитела памяти к SARS-CoV-2 у них изначально отсутствуют [23]. Когда же вирус попадает в организм ребенка первоначально, начинаются синтез и секреция IgM, которые в свою очередь способствуют более интенсивной выработке долговременного IgG, не вызывая при этом манифестной инфекции [24]. Вероятно, не случайно, что максимальная доля серопревалентных лиц с бессимптомной формой заболевания выявлена именно среди детей (см. табл. 3).
В остальных возрастных группах серопозитивных волонтеров доля бессимптомных лиц варьировала в пределах, близких к среднепопуляционному показателю.
Таким образом, исследование серопревалентности населения Владимирской области показало, что уровень популяционного иммунитета в еще не достиг порогового уровня, при котором наблюдается резкое снижение заболеваемости, в связи с чем необходимы дальнейшие эпидемиологические меры, направленные на защиту населения от COVID-19.
Выводы
1. Коллективный иммунитет совокупного населения Владимирской области составил 10,7%.
2. Максимальный уровень коллективного иммунитета установлен у детей 1–17 лет (19,9%), при этом у детей младшего возраста (1–6 лет) он составил 25,2%. В группе лиц старше 70 лет доля серопозитивных составила 19,2%.
3. При наличии контактов с больными COVID-19 сероконверсия увеличивается примерно в 1,5 раза.
4. Среди лиц, перенесших COVID-19, доля серопозитивных достигает 57,1%.
5. У лиц с положительным результатом ПЦР-анализа, полученным ранее, антитела выявляют в 42,3% случаев.
6. Среди сероположительных жителей Владимирской область в целом доля бессимптомных форм составляет 87,6%.


