Вспышки менингококковой инфекции (МИ) возникают во многих регионах мира, но наиболее часто регистрируются в так называемом менингитовом поясе – районе Африки к югу от Сахары с населением более 400 млн чел., простирающимся от Сенегала до Эфиопии [1]. Для него характерны эпидемии в течение сухого сезона с декабря по июнь (годовые показатели заболеваемости часто составляют 10–100 случаев на 100 тыс. населения), а взрывные эпидемии происходят в 8–12-летних циклах, когда показатели заболеваемости могут превышать 1000 случаев на 100 тыс. населения. Описан факт наибольшего риска возникновения эпидемий в период низкой абсолютной влажности, при возникновении пыльных бурь, при высоких показателях температуры воздуха. Изменение климатических и погодных условий может увеличивать географический диапазон стран, подверженных риску возникновения МИ [2]. Исторически эпидемии в «менингитовом поясе» были в основном связаны с Neisseria meningitidis (N. meningitidis) серогруппы А. С момента введения в 2010 г. конъюгированной вакцины против МИ, вызванной штаммами менингококка серогруппы А, эпидемии в «менингитовом поясе» прекратились, хотя эпидемии, вызванные менингококком других серогрупп, продолжаются [3]. Заболеваемость МИ в других регионах варьирует во времени и в зависимости от серогруппы менингококка [4]MenA in the African meningitis belt. В некоторых странах за пределами «менингитового пояса» наблюдались периоды, когда показатели заболеваемости превышали 4 на 100 тыс. населения, сохранявшиеся в течение нескольких лет, но чаще всего достигали 2 на 100 тыс. населения или менее [5, 6]. Самые низкие показатели заболеваемости МИ зафиксированы в Азии [4]MenA in the African meningitis belt.
Факторы риска по заболеванию МИ включают переуплотнение в помещении при совместном проживании, активное курение, воздействие дыма, тесный контакт с больным, ВИЧ-инфекцию, асплению, дефицит комплемента и др. Хорошо известны эпидемии, связанные с паломническими поездками (хадж и умра). Инфекции, вызванные вирусом гриппа и респираторно-синцитиальным вирусом, могут предрасполагать к инвазивному менингококковому заболеванию [7].
В XX веке в нашей стране зарегистрированы 2 эпидемии МИ с пиком заболеваемости в 1931 и 1970 гг. Н.Н. Костюкова и соавт. [8] представили исторические периоды подъема заболеваемости МИ. Первый начался в 1902 г. и продолжался до окончания Великой Отечественной войны с наивысшими показателями заболеваемости в Татарстане, в Средне-Волжском районе, на Кавказе, в Белоруссии и на Украине. По всей вероятности, повышенная заболеваемость была вызвана N. meningitidis серогруппы А, как и во многих странах мира в тот же период. Второй подъем начался с 1968 г. в Липецке и Курске среди подростков, приехавших на учебу в СССР из Вьетнама. Было установлено, что инфекцию привезли подростки, заразившись либо на территории своей страны, либо на территории Китая, через который они ехали с остановками. После пересечения китайской границы подростков пересадили в тесные закрытые вагоны, которые стали своего рода инкубаторами инфекции. В Чите был снят больной с подозрением на менингит, а к местам конечных точек следования прибыло много инфицированных. Через 25 лет в Институте Макса Планка в Берлине Mark Achtman с сотрудниками подтвердили, что распространившиеся после 1968 г. в нашей стране штаммы серогруппы А были генетически идентичны штаммам, вызвавшим большую эпидемию в Китае в 1965–1969 гг., и относились к сиквенс-типу ST-5 генетической субгруппы III по данным мультилокусного энзимэлектрофореза (Multiple Loci Enzyme Electrophoresis) [9]. Инфекция распространилась в средней полосе территории СССР и в Москве. От больных выделяли менингококк серогруппы А. Подъем продолжался до 1991 г. В него были вовлечены все административные территории страны. Далее регистрировали несколько эпизодов осложнения эпидемической ситуации по МИ в стране. Так, в 1996 г. в Москве произошел подъем заболеваемости МИ, вызванной N. meningitidis серогруппы А, среди торговцев из Вьетнама [10]. Штаммы менингококка принадлежали к генетической субгруппе III, однако детальное изучение показало их отличие от штаммов, вызвавших эпидемию в 1970-х гг. во многих странах мира: они относились к сиквенс-типу ST-7. Подъем заболеваемости в Москве был купирован благодаря вакцинации групп риска (детей от 1 года до 7 лет). Небольшие подъемы заболеваемости в 2003 и 2009 гг. в Москве были связаны с увеличением доли серогруппы А генетической субгруппы X, относящихся к сиквенс-типу ST-75 [9, 11]. Для купирования распространения заболевания в 2003 г. проведена вакцинация детей, а в 2009 г. – вакцинация приезжих рабочих-строителей в соответствии с Постановлением № 3 Главного Государственного санитарного врача по городу Москве от 17 апреля 2009 г. «Об усилении мероприятий по профилактике менингококковой инфекции в Москве».
По данным Российского референс-центра по мониторингу за бактериальными менингитами (далее – РЦБМ), функционирующего на базе Центрального НИИ эпидемиологии Роспотребнадзора (ЦНИИЭ, Москва), в Российской Федерации впервые за 13-летний период (с 2004 г.) прерван этап устойчивого снижения заболеваемости генерализованной формой МИ (ГФМИ). В 2017 г. показатель заболеваемости вырос, составив 0,48 на 100 тыс. населения против 0,45 на 100 тыс. населения в 2016 г. В 2018 и 2019 гг. он продолжил расти до 0,56 и 0,6 на 100 тыс. населения соответственно. Средний прирост за период 2016–2019 гг. составил 10,4%, абсолютный прирост – 0,15 на 100 тыс. населения.
На фоне постепенного подъема заболеваемости МИ на территории страны в марте–июне 2019 г. возникла крупная вспышка в Новосибирске. По ретроспективным данным Управления Роспотребнадзора по Новосибирской области и Центра гигиены и эпидемиологии в Новосибирской области, за последние 17 лет заболеваемость МИ в Новосибирской области снизилась в 6,2 раза. В последние годы регистрировали спорадические, не связанные друг с другом случаи заболеваний (в 2016 г. – 7 случаев, в 2017 г. – 11, в 2018 г. – 15). В динамике за период с 2010 по 2018 г. в Новосибирской области почти в равных соотношениях циркулировали штаммы менингококка 3 основных серогрупп (А, В, С) с некоторым преобладанием серогруппы В (в 2010 г. – 43%, в 2011 г. – 68%, в 2012 г. – 36%, в 2013 г. – 30%, в 2015 г. – 40%). Выявлен высокий уровень циркуляции штаммов менингококка серогруппы А, при этом в отдельные годы он занимал лидирующую позицию (в 2014 г. – 48%, в 2017 г. – 47%).
Активная регистрация необычно высокого числа случаев началась неожиданно, когда за 4 месяца 2019 г. (с 27.03 по 17.06) зарегистрировано 62 случая ГФМИ, показатель заболеваемости в 3 раза превысил показатель за тот же период 2018 г. – 0,65 и 0,22 на 100 тыс. населения соответственно. Осложнение эпидемической ситуации и концентрация почти всех случаев отмечены в Новосибирске.
Цель исследования – анализ эпидемиологических проявлений вспышки МИ, молекулярно-биологическая характеристика возбудителя, установление предположительных причин ее возникновения и описание мер, предпринятых для ее купирования.
Материалы и методы
Расследование вспышечной заболеваемости МИ в Новосибирске проведено в июне 2019 г. специалистами Управления Роспотребнадзора по Новосибирской области Министерства здравоохранения Новосибирской области при участии специалистов РЦБМ и Центра молекулярной диагностики и эпидемиологии (на базе ЦНИИЭ).
Проанализированы 54 карты эпидемиологического обследования очага инфекционного заболевания (форма N 357-у, утверждена Приказом Минздрава СССР 04.10.80 № 1030)
Госпитализацию больных с подозрением на МИ проводили в ГБУЗ Новосибирской области «Городская инфекционная клиническая больница № 1» (ГИКБ № 1) и ГБУЗ Новосибирской области «Детская городская клиническая больница № 3» (ДГКБ № 3).
Для исследования биологического материла (спинномозговой жидкости и крови) от больных были выполнены бактериологический посев, бактериоскопическое исследование, экспресс-диагностика с помощью реакции латекс-агглютинации, ПЦР-диагностика для определения ДНК основных возбудителей гнойных бактериальных менингитов. Для тестирования биологического материала в РЦБМ были направлены образцы от 20 больных. Лабораторное подтверждение МИ в РЦБМ устанавливали с помощью набора реагентов «АмплиСенс® NSH-FL», серогруппирование N. meningitidis – с помощью набора «АмплиСенс® NmABCW-FL» [12].
Для определения активности скрытого звена в эпидемическом процессе МИ исследовали широту распространения менингококкового носительства в индикаторных группах Новосибирска (трудовые мигранты, работающие на рынке; дети трудовых мигрантов; студенты Новосибирского государственного аграрного университета). Выявление и идентификацию носоглоточных штаммов менингококка проводили микробиологическими, серологическими1 и молекулярно-биологическими методами [12, 13]. Антигенные и генетические характеристики штаммов определены согласно международным требованиям к типированию бактерий вида N. meningitidis [11–14]. Бактериальная ДНК секвенирована методом Сэнгера с использованием реагентов и оборудования фирмы Applied Biosystems (США). Определены 3 поверхностных вариабельных фрагмента белков наружной мембраны PorA и FetA, аллельный профиль, сиквенс-тип и принадлежность к известным клональным комплексам [15].
Поскольку использование рекомендованной ранее [13] «классической» 7-локусной схемы МЛСТ в сочетании с характеристикой 3 антигенных фрагментов не всегда позволяет дискриминировать возбудителей ГФМИ, имеющих разное для наблюдаемой территории происхождение, с целью определения эпидемиологических закономерностей их распространения были получены полногеномные последовательности штаммов N. meningitidis. Секвенирование проводили с помощью платформы HiSeq1500 (Illumina, США). Использованы протоколы для пробоподготовки и сборки полногеномных последовательностей, описанные ранее [14, 16]. Для проведения МЛСТ-анализа по «основному геному» (core genome MLST – cgMLST) [17] использованы программные возможности Интернет-ресурса PubMLST.org [18]. Генетические взаимоотношения изученных штаммов визуализированы с использованием программы SplitsTree (версия 4.14.6) [19].
Накопление, корректировку, систематизацию исходной информации и визуализацию результатов осуществляли в электронных таблицах Microsoft Office Excel 2011. Для статистической обработки применили программу IBM SPSS Statistics v.26.
Результаты и обсуждение
Начиная с марта 2019 г. в течение 4 мес., в Новосибирске были госпитализированы 62 заболевших с диагнозом ГФМИ. За период 27.03 по 05.06 число заболеваний МИ постепенно нарастало, с 06 по 11.06 произошел резкий подъем заболеваемости, когда за 6 дней был зарегистрирован 21 случай ГФМИ, и далее с 12 по 17.06 возникло еще 5 случаев. При вспышке МИ в эпидемический процесс в основном оказались вовлечены 48 (77%) трудовых мигрантов из Таджикистана, при этом доля жителей Новосибирска была в 3,4 раза меньше – 14 (23%) чел. (рис. 1).
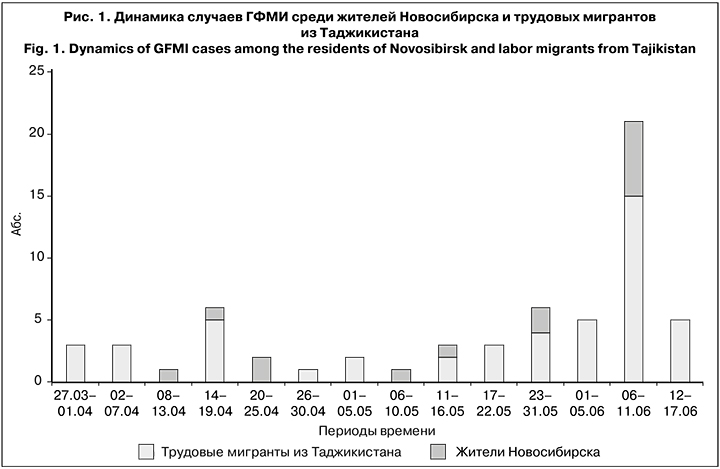
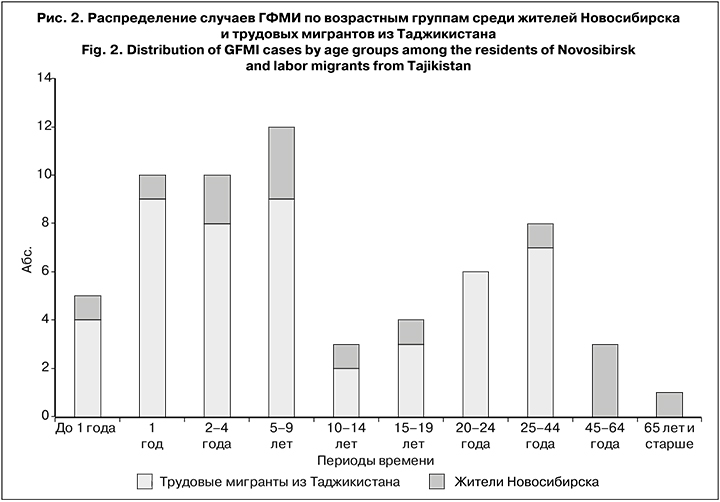
Возраст заболевших находился в диапазоне от 6 мес. до 69 лет. Большинство составили дети до 15 лет (40 случаев; 65%). Среди заболевших мигранты из Таджикистана преобладали почти во всех возрастных группах, за исключением лиц старше 45 лет (рис. 2). Лица мужского пола преобладали как среди детей до 15 лет (60%), так и среди взрослых (77%).
Случаи заболеваний регистрировали в 9 районах города, в 3 пригородных сельских районах, и 1 случай возник в г. Искитим. Наиболее пораженными оказались Октябрьский и Дзержинский районы Новосибирска (16 и 14 случаев соответственно), в Калининском районе выявлено 7 случаев заболеваний, в Кировском – 5. В остальных районах города регистрировали единичные случаи заболеваний.
За время вспышки выявлено 6 очагов с групповыми случаями ГФМИ: 2 очага с 4 случаями, 1 очаг с 3 случаями и 3 очага с 2 случаями заболевания.
Наиболее распространенной оказались смешанная форма ГФМИ (менингококкцемия и менингит) – 27 (44%) случаев и менингококкокцемия – 22 (35%) случая. В 5 (8%) случаях поставлен диагноз «менингит», в 8 (13%) случаях форму ГФМИ уточнить не удалось.
В ГИКБ № 1 и ДГКБ № 3 бактериологические лаборатории проводят бактериоскопическое исследование и посевы ликвора и крови, выполняют экспресс-диагностику с помощью реакции латекс-агглютинации, ПЦР-диагностику на выявление ДНК основных возбудителей гнойных бактериальных менингитов. Лабораторно обследовано 62 пациента, у них подтверждено 37 (60%) случаев. Удалось установить серогруппу 32 штаммов, из них 29 (91%) определены как N. meningitidis серогруппы А. В 1 случае заболевание было вызвано N. meningitidis серогруппы В, в 2 – серогруппы С.
Для тестирования и ретестирования биологического материала в РЦБМ доставлены образцы от 20 больных. Результаты лабораторной диагностики этих случаев, полученные в ГИКБ № 1 и ДГКБ № 3 Новосибирска, и результаты их тестирования в РЦБМ оказались идентичными.
Во время вспышки 1 случай из 62 закончился летальным исходом: мужчина 69 лет, житель. Новосибирска, со смешанной формой ГФМИ, вызванной N. meningitidis серогруппы А. Таким образом, показатель летальности в период эпидемического подъема составил 2%.
Каждый случай заболевания сопровождала карта эпидемиологического обследования очага инфекционного заболевания. В ходе расследования вспышки проанализировано 54 карты, 8 карт не предоставлено.
Наличие переуплотнения в местах проживания заболевших указано в 23(43%) случаях. К социальному нормативу 18 м2 и выше на 1 чел. удалось отнести жилищные условия только 4 заболевших. В остальных случаях квадратура варьировала от 3,6 м2 (5 чел. в одной комнате) до 17,2 м2 (15 чел. в 12 комнатах). Таким образом, 93% больных, зарегистрированных во время вспышки, проживали в условиях переуплотнения.
В 15 (28%) случаях заболевшие ГФМИ имели в своем окружении болеющих ОРВИ (2 случая), назофарингитом (4), ГФМИ (5), ОРВИ и ГФМИ (4).
Большинство заболевших относились к трудовым мигрантам из Таджикистана (48 чел.). Установлено, что 8 из них посещали мечеть в Дзержинском районе Новосибирска, а 15 (1 женщина и 14 детей) имели в своем окружении лиц, посещающих эту мечеть (папа, дядя, сосед в квартире). Еще 1 заболевший, мальчик 9 лет, имевший контакт с посещавшим мечеть, был жителем Новосибирска. Таким образом, 24 (44%) из 54 заболевших (44%) были связаны с посещением одной мечети самостоятельно или через свое близкое окружение.
В ближайшем окружении 17 заболевших мигрантов из Таджикистана были лица, работающие в местах организованной торговли (вещевой и фруктовый рынки, торговый центр). Еще 1 заболевшая (женщина, русская, 60 лет) самостоятельно посещала рынок, и у 1 заболевшей (девочка, русская, 3 года) папа имел по работе контакт с мигрантом из Таджикистана – работником торгового центра. Таким образом, 19 (35%) заболевших были связаны с посещением мест организованной торговли своим ближайшим окружением (родственники/соседи по квартире) или посещали их самостоятельно.
Для определения активности скрытого звена в эпидемическом процессе МИ проведено исследование широты распространении менингококкового носительства в индикаторных группах Новосибирска. Получены биологические образцы (мазок из носоглотки в транспортной среде Amies) от 298 лиц: 112 трудовых мигрантов, работающих на рынке (19–69 лет); 86 детей трудовых мигрантов (6 –18 лет) и 100 студентов НГАУ (17–34 года). Общий уровень носительства составил 1,7% (5 носителей из 298 обследованных). При этом среди детей трудовых мигрантов и студентов НГАУ не выявлено ни одного носителя, а среди трудовых мигрантов – 5 (4,5%) в возрасте от 20 до 45 лет. Все выделенные от носителей штаммы менингококка отнесены к серогруппе А.
4 образца, поступившие в РЦБМ из Новосибирска (суспензии культур N. meningitidis серогруппы А, выделенные из крови и спинномозговой жидкости больных ГФМИ), исследованы методом мультилокусного секвенирования-типирования (МЛСТ). Исследованные штаммы имели сиквенс-тип ST-75, входящий в клональный комплекс «ST-1 complex/subgroup I/II» (cc1), представителей которого часто выявляли в России (на территории Москвы) [11, 15].
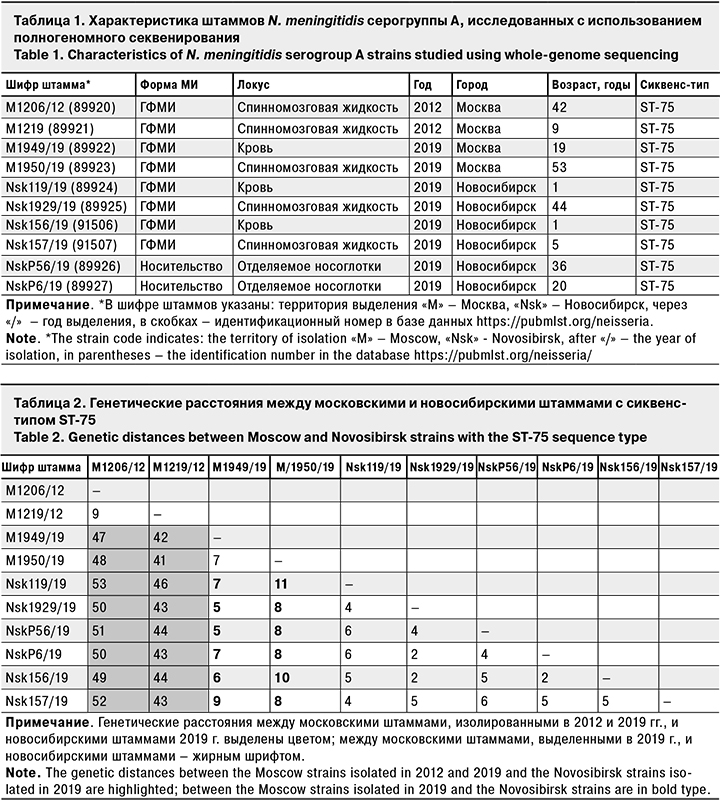
Для сравнения московских и новосибирских штаммов проведено их полногеномное исследование (2 московских штамма от больных 2012 г., 2 московских штамма от больных 2019 г., 4 новосибирских штамма от больных 2019 г. и 2 новосибирских штамма от носителей 2019 г.) (табл. 1).
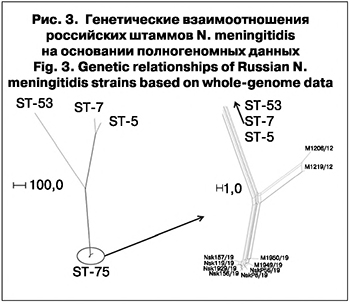 Для всех штаммов установлена принадлежность к сиквенс-типу ST-75 с одинаковыми антигенными характеристиками: A: P1.5-2,10: F3-5: ST-75 (cc1). Ранее нами было показано, что циркуляция таких штаммов характерна для Москвы и не связана с подъемами заболеваемости [11, 12, 15]. Из-за недостаточной дискриминирующей способности рекомендованной «классической» 7-локусной схемы типирования для идентификации эпидемиологически связанных случаев ГФМИ, вызванных штаммами с сиквенс-типом ST-75, был проведен анализ их генетических взаимоотношений на основании полногеномных данных [16, 17]. Для сравнения были использованы штаммы N. meningitidis серогруппы А того же сиквенс-типа, выделенные на территории Москвы от больных ГФМИ в 2012 и 2019 гг. (табл. 2). При анализе и визуализации данных использованы штаммы N. meningitidis серогруппы А с сиквенс-типами ST-5, ST-7 и ST-53, выделенные на территории Российской Федерации ранее, полногеномные последовательности которых были доступны через PubMLST (идентификационные номера в базе данных https://pubmlst.org/neisseria 451, 34857 и 61349 соответственно). Анализ генетических взаимоотношений охарактеризованных штаммов на основании полногеномного секвенирования представлен на рис. 3.
Для всех штаммов установлена принадлежность к сиквенс-типу ST-75 с одинаковыми антигенными характеристиками: A: P1.5-2,10: F3-5: ST-75 (cc1). Ранее нами было показано, что циркуляция таких штаммов характерна для Москвы и не связана с подъемами заболеваемости [11, 12, 15]. Из-за недостаточной дискриминирующей способности рекомендованной «классической» 7-локусной схемы типирования для идентификации эпидемиологически связанных случаев ГФМИ, вызванных штаммами с сиквенс-типом ST-75, был проведен анализ их генетических взаимоотношений на основании полногеномных данных [16, 17]. Для сравнения были использованы штаммы N. meningitidis серогруппы А того же сиквенс-типа, выделенные на территории Москвы от больных ГФМИ в 2012 и 2019 гг. (табл. 2). При анализе и визуализации данных использованы штаммы N. meningitidis серогруппы А с сиквенс-типами ST-5, ST-7 и ST-53, выделенные на территории Российской Федерации ранее, полногеномные последовательности которых были доступны через PubMLST (идентификационные номера в базе данных https://pubmlst.org/neisseria 451, 34857 и 61349 соответственно). Анализ генетических взаимоотношений охарактеризованных штаммов на основании полногеномного секвенирования представлен на рис. 3.
В табл. 2 представлена матрица генетических расстояний между охарактеризованными штаммами – количество несовпадений в генетических локусах (1441), образующих «основной геном» N. meningitidis.
Установлено невысокое генетическое разнообразие циркулирующих на наблюдаемой территории штаммов с сиквенс-типом ST-75 и антигенным профилем A: P1.5-2,10: F3-5: максимальное количество несовпадений в «основном геноме» между ними не превышает 4%. Генетическое расстояние между штаммами, изолированными в 2019 г. в Москве и Новосибирске (как от больных ГФМИ, так и от носителей), было минимально: количество несовпадений в «основном геноме» составляет 5–11 или 0,35–0,76%, по сравнению со штаммами, изолированными в Москве в 2012 г., с которыми минимальное количество несовпадений составляет более 40 или около 3%. Это позволяет сделать вывод о том, что вспышка ГФМИ в Новосибирске обусловлена группой штаммов, генетически наиболее близких к московским штаммам, выделенным от больных ГФМИ в 2019 г. Дополнительный анализ локусов, образующих «основной геном», не выявил аллелей генов, ассоциированных с резистентностью к антибиотикам.
Возникновение вспышки МИ в Новосибирске обусловлено рядом факторов. Она началась с возникновения очага сразу с 3 случаями ГФМИ. Так, 27.03.2019 заболела девочка-таджичка Х.А. (1 год), проживавшая в Дзержинском районе города, и ее брат (3 года). Из карты девочки следует, что ее семья принимала гостей, и из числа гостей 28.03 ГФМИ заболела девочка-таджичка З.О. (1 год), которая проживала в Калининском районе Папа девочки З.О. посещал мечеть. Папы девочек работали на одном рынке, кроме того, папа девочки З.О. посещал мечеть.
Установлено, что 05.05 мусульмане отмечали наступление месяца Рамадана, когда верующие исполняют предписание ежедневной молитвы гораздо строже, чем в другое время. Выполнялись религиозные практики, подразумевающие длительное непрерывное нахождение в мечети. 04.06 Рамадан завершился исламским праздником Ураза-байрам. По традиции в этот день следует посетить родственников, а также устроить праздник у себя дома. Так, семья одного заболевшего ГФМИ накануне приняла дома одновременно около 30 гостей. Сразу после праздника начался резкий подъем заболеваемости: за 5 дней (с 06 по 11.06) госпитализирован 21 заболевший из 6 районов города. Предпринятые меры по локализации и ликвидации вспышки позволили снизить заболеваемость, тем не менее за период с 12. по 17.06 было зарегистрировано еще 5 случаев заболеваний.
Таким образом, в рамках настоящей вспышки в той или иной степени реализовались известные факторы риска МИ [7], кроме факта курения, информация по которому в картах эпидемиологического обследования отсутствовала. Можно предположить, что наличие вредных привычек имело место либо в форме курения сигарет, либо в виде употребления широко используемых в странах Средней и Центральной Азии некурительных табачных изделий, раздражающих слизистую оболочку полости рта и носоглотки.
Известно, что заболеваемость МИ характеризуется периодичностью в 10–20–30 лет, наивысший подъем вызывает менингококк серогруппы А, а за отдельные подъемы и вспышки – серогруппы В и С. Как и при любой инфекции с капельным механизмом передачи, интенсификация эпидемического процесса МИ зависит от деятельности человека: активной урбанизации, развития транспортных связей [8]. Для Российской Федерации в XX веке были характерны 30-летние периоды эпидемиологического благополучия по МИ. В апреле 2017 г. на Конгрессе инфекционистов в Москве сотрудниками РЦБМ был представлен прогноз заболеваемости МИ, основанный на анализе многолетней цикличной смены периодов ее эпидемического спада и подъема. Прогнозировался очередной подъем заболеваемости к 2020 г. Действительно, с 2017 г. показатель заболеваемости начал возрастать. Таким образом, первая в XXI веке вспышка МИ в Российской Федерации предположительно явилась результатом периодической многолетней интенсификации эпидемического процесса МИ и влияния ряда факторов риска МИ, реализованных и поддерживаемых особенностями жизни и быта трудовых мигрантов из Таджикистана.
Для купирования вспышки был проведен комплекс профилактических и противоэпидемических мероприятий в соответствии с санитарными правилами СП 3.1.3542-18 «Профилактика менингококковой инфекции». На 20.06 по месту проживания в очагах МИ выявлено 854 контактных с больными лиц, которым назначены осмотр врачом, химиопрофилактика и вакцинация. Среди контактных с использованием 2 вакцин (менингококковой полисахаридной конъюгированной серогрупп А, С, Y и W и полисахаридной менингококковой серогруппы А) привито 754 (89%) чел. В 7 детских образовательных организациях выявлено 322 контактных лица, из которых привито 298 (96%). Специалисты Управления Роспотребнадзора по Новосибирской области и Министерства здравоохранения Новосибирской области проводили иммунизацию по эпидемическим показаниям трудовых мигрантов, в том числе посещающих мечеть, и в местах массового их скопления. Общее число привитых на 20.06.2019 составило 1346 чел. (67% от числа подлежащих иммунизации). Проводили иммунизацию детей в возрасте 1–8 лет в первую очередь в Октябрьском и Дзержинском районах города. Всего на 20.06 привили 4559 чел. , в том числе 3167 в организованных детских коллективах и 1392 неорганизованных детей. Также по согласованию с имамами было предписано ограничить посещение мечетей. Было принято решение организовать комиссионные обследования условий проживания трудовых мигрантов, обеспечив их максимальное разуплотнение. Предпринятые меры в очагах инфекции и проведение плановой профилактической иммунизации в когорте контингентов риска (привито более 40 000 чел.) позволили купировать вспышку МИ в Новосибирске. После 17.06 и до конца 2019 г. зарегистрировано всего 3 случая ГФМИ. Все случаи возникли в июле 2019 г. и были обусловлены менингококком серогруппы А.
Заключение
В Новосибирске выявлен эпидемический подъем заболеваемости МИ. Общее число заболевших за 4 месяца 2019 г. составило 62 чел., большинство из них – трудовые мигранты из Таджикистана. В этиологии заболевания преобладали штаммы N. meningitidis серогруппы А (91%). Вспышка обусловлена группой штаммов c сиквенс-типом ST-75 и антигенным профилем A: P1.5-2,10: F3-5, которые ранее выявляли на территории Российской Федерации. Они вызывали спорадическую заболеваемость и не были причиной вспышек и эпидемий. В ближайшем окружении заболевших проявились факторы риска МИ: переуплотнение в местах проживания; окружение (родственники/соседи в одной квартире) с ОРВИ и назофарингитом; скученность и интенсификация общения во время религиозного поста и праздника; семейный анамнез, включающий больного ГФМИ, а также контакт с больным ГФМИ вне семьи.
Учитывая продолжающийся рост заболеваемости МИ в стране, а также наличие условий для возникновения вспышки МИ, не исключена угроза нового эпидемического подъема заболеваемости на территории Российской Федерации среди мигрантов. В этой связи необходимы:
1. Мониторинг проявлений эпидемического процесса МИ и выявление предвестников осложнения эпидемической ситуации в субъектах Российской Федерации (в соответствии с п. 6.4. СП 3.1.3542-18).
2. Реализация комплекса профилактических и противоэпидемических мероприятий, предусмотренного СП 3.1.3542-18 при выявлении предвестников осложнения эпидемической ситуации.
3. Внесение изменений в календарь профилактических прививок по эпидемическим показаниям (Приказ 125н от 21.03.2014, действующая последняя редакция приказа № 175н от 13.04.2017) в соответствии с формулировками СП 3.1.3542-18.
4. Рассмотрение вопроса о включении прививок против МИ детям первых лет жизни в национальный календарь профилактических прививок.
5. Исследование образцов биологического материала и штаммов, полученных от больных при возникновении вспышек ГФМИ, с помощью микробиологических и молекулярно-биологических методов для определения комплекса их антигенных и генетических свойств.
6. Активное применение метода высокопроизводительного секвенирования, позволяющего получать исчерпывающую информацию о возбудителе ГФМИ для выявления эпидемиологических связей и поиска опасных клонов.


