В настоящее время методики лечения COVID-19 различаются не только в общемировом масштабе, но и в рамках субъектов, а иногда и отдельных учреждений конкретных муниципальных образований России. Несмотря на накопленный опыт весенней волны пандемии, противоречия в тактике диагностики и лечения инфекции, вызванной SARS-СoV-2, не стали менее значимыми. Один из наиболее острых вопросов заключается в том, как оптимизировать лечение пациентов с нетяжелыми формами COVID-19, какую этиотропную терапию назначить, чтобы она была и эффективной, и безопасной.
Так например, активно назначавшиеся на начальных этапах поиска этиотропного лечения SARS-CoV-2 препараты гидроксихлорохина демонстрируют значительную кардиотоксичность [1]. Тем не менее на сегодняшний день золотым стандартом терапии вирусных инфекций является своевременное (с первых дней болезни) включение этиотропных лекарственных средств. Раннее назначение противовирусных препаратов способствует более быстрой элиминации вируса, предупреждает возникновение хронических очагов вирусной инфекции и развитие в более поздние периоды заболевания осложнений, порой фатальных, обусловленных ошибочной тактикой ведения больного, а именно – отказом лечащего врача от назначения противовирусной терапии или ее несвоевременным введением в протокол лечения [2]. На сегодняшний день доказано, что медленные (хронические вирусные) инфекции способствуют поражению жизненно важных органов, вызывая латентно протекающие и труднодиагностируемые миокардиты, энцефалиты, васкулиты и другие повреждения органов и систем [3, 4].
В то же время избыточное назначение лекарственных средств также способно привести к неблагоприятному течению болезни за счет медикаментозно опосредованного влияния на организм, например, сочетанное применение гидроксихлорохина с азитромицином [5].
В качестве этиотропного препарата, обладающего известным механизмом прямого противовирусного действия и одновременно благоприятным профилем безопасности, был исследован фавипиравир. Он представляет собой химическое соединение, относящееся к нуклеозидным аналогам. При попадании внутрь клетки фавипиравир преобразуется внутриклеточными ферментами в активный метаболит фавипиравир рибозил трифосфат (РТФ). В отношении РНК-полимеразы вируса гриппа типа А фавипиравир-РТФ выступает более эффективным субстратом по сравнению с АТФ и ГТФ, превосходя активность в отношении их в 30 и 19 раз соответственно. При этом фавипиравир практически не влияет на активность человеческих ДНК-полимераз α, β и γ, а ингибирующая концентрация (IC50) фавипиравира в отношении человеческой РНК-полимеразы II более чем в 1000 раз превосходит IC50 для РНК-полимеразы вируса пандемического гриппа pdm.
Фармакокинетические показатели фавипиравира оценивали в поуляции пациентов, включенных в подгруппу исследования фармакокинетики (ФК).
Цель исследования – обоснование необходимости и безопасности применения фавипиравира в лечении нетяжелых форм COVID-19 у взрослых пациентов.
В качестве конечных точек исследования сравнивали быстроту клинического улучшения по шкале ВОЗ и сроки элиминации вируса, наличие или отсутствие нежелательных явлений (НЯ), вызванных непосредственно принимаемыми препаратами, а также их выраженность.
Материалы и методы
В общей сложности в исследовании приняли участие 168 пациентов. Все они подписали информированное согласие до включения в исследование. Пациенты были рандомизированы в группу исследуемой терапии и группу сравнения в соотношении 2:1 соответственно.
Критерии включения в исследование: легкая и среднетяжелая степень тяжести заболевания, возраст от 18 до 65 лет, выраженность патологии на КТ органов грудной клетки (КТ-0 – КТ-1 и КТ-2 – КТ-3).
Пациенты основной группы на протяжении 10 дней получали терапию препаратом «КОРОНАВИР» (фавипиравир) перорально: в 1-й день – нагрузочную дозу (по 1800 мг с интервалом в 12 ч, то есть 2 раза в сутки), со 2-го по 10-й день – по 800 мг с интервалом в 12 ч (то есть 2 раза в сутки). Выбранный режим терапии был основан на литературных данных о ФК препарата. Фавипиравир хорошо всасывается после приема внутрь. Максимальная концентрация в плазме крови достигается менее чем через час после приема натощак, однако при применении его в высоких дозах (1200 мг и более) время достижения максимальной концентрации увеличивается. Более 90% введенной дозы выделяется через почки, таким образом, биодоступность у здоровых добровольцев можно считать более 90%.
Фавипиравир – препарат со сложной нелинейной ФК. После многократных доз время достижения максимальной концентрации в плазме и период полувыведения увеличивались. При сравнении ФК в популяции японских и американских добровольцев у американских добровольцев концентрация препарата в плазме крови была примерно на 50% ниже, чем у японских. Основным ферментом, участвующим в элиминации фавипиравира, является альдегидоксидаза, которая превращает его в неактивный метаболит М1. Сложная дозозависимая ФК фавипиравира, вероятно, обусловлена насыщением и/или аутоингибированием основного ферментативного пути, поскольку было показано, что препарат ингибирует альдегидоксидазу in vitro. В настоящем исследовании схема применения фавипиравира была выбрана на основании данных по ФК, полученных в США, поскольку ожидалось включение преимущественно европеоидной популяции пациентов. Запланированный режим терапии был основан на дозах препарата, показавших максимальную эффективность при лечении гриппа.
В группе сравнения пациенты получали рекомендованную «стандартную» этиотропную терапию в соответствии с актуальной версией 6 Временных методических рекомендаций Минздрава России «Профилактика, диагностика и лечение коронавирусной инфекции (COVID-19)», действовавшей на момент проведения исследования [6]: умифеновир применяли по 1 капсуле (200 мг) 4 раза в сутки перорально в течение 5 дней, рекомбинантный ИФН-α-2b (капли назальные 10 000 МЕ/мл) – по 3 капли в каждый носовой ход 5 раз в день в течение 5 дней. При необходимости терапия могла быть продолжена на срок до 10 дней. Гидроксихлорохин применяли по следующим схемам:
- схема 1 – в 1-й день 800 мг (по 400 мг 2 раза в сутки), далее 400 мг (по 200 мг 2 раза в сутки) в течение 6–8 дней (применение в условиях стационара при возможности мониторинга интервала QT);
- схема 2 – в 1-й день 400 мг (по 200 мг 2 раза в сутки), далее 200 мг (по 100 мг 2 раза в сутки) в течение 6–8 дней (применение в амбулаторной практике или при отсутствии возможности мониторинга интервала QT).
В качестве сопутствующей терапии пациентам обеих групп в зависимости от состояния назначали жаропонижающие, мукоактивные, бронхолитические и другие препараты; антикоагулянты, а также антибактериальную терапию при присоединении вторичной инфекции.
У всех пациентов на протяжении исследования оценивали эффективность терапии: клинический статус; субъективную симптоматику; наличие вируса SARS-CoV-2 (методом ПЦР) в мазке из верхних дыхательных путей; температуру тела; показатели АД, ЧСС и ЧДД; насыщение крови кислородом по данным пульсоксиметрии (SpO2); результаты КТ органов грудной клетки.
Безопасность проводимого лечения оценивали на основании жалоб пациентов, жизненно важных показателей, результатов лабораторных анализов и ЭКГ. Результаты лабораторных и инструментальных исследований оценивали на скрининге, далее – на 5-й, 14-й и 28-й день. Лабораторные исследования включали клинический анализ крови (гемоглобин; число эритроцитов, лейкоцитов, нейтрофилов, лимфоцитов, тромбоцитов; СОЭ); биохимический анализ крови (глюкоза, АЛТ, АСТ, ЛДГ, билирубин общий, креатинин, КФК, ферритин, лактат, мочевая кислота); анализ на С-реактивный белок; коагулограмму (АЧТВ, протромбиновое время, фибриноген, D-димер); общий анализ мочи. Инструментальные исследования включали КТ органов грудной клетки и ЭКГ.
Средний возраст пациентов в основной группе составил 41,7 ± 10,6 года, в группе сравнения – 42,0 ± 10,4 года. В легкой форме заболевание протекало у 25,0% пациентов в основной группе и у 26,8% в группе сравнения, в среднетяжелой – у 75,0 и 73,2% соответственно.
Эффективность фавипиравира оценивали по времени наступления улучшения клинического статуса и достижения элиминации вируса (отсутствие SARS-CoV-2 по результатам 2 последовательных ПЦР-тестов, выполненных с интервалом не менее 24 ч).
Фармакокинетические показатели препарата рассчитывали после однократного и многократного его применения. На основании данных о концентрации были рассчитаны максимальная концентрация (Cmax); площадь под кривой концентрация/время за 12 ч от момента приема препарата (AUC(0-12); время достижения максимальной концентрации (Tmax); время полувыведения препарата (T1/2); константа элиминации (Kel) и клиренс (Cl). Оценивали также частоту НЯ/серьезных НЯ. Показатели анализировали, применяя методы описательной статистики. Использовали t-критерий Стьюдента, U-критерий Манна–Уитни, Т-критерий Вилкоксона, параметрический дисперсионный анализ (ANOVA) для повторных измерений, критерий Фридмана и критерий Фишера.
Результаты
Частота элиминации вируса была значимо выше в основной группе: на 3-й день – у 71,4% пациентов vs 57,1% в группе сравнения, на 5-й день – у 81,2% vs 67,9% соответственно. Медиана времени до клинического улучшения по Порядковой шкале клинического улучшения ВОЗ в основной группе составила 6,0 (4,00; 9,25) дней, в группе сравнения – 10,0 (5,0; 21,0) дней. Продемонстрированное преимущество в 4 дня статистически достоверно (р < 0,01) и является клинически значимым, учитывая общую тяжесть состояния пациентов.
На основании результатов проведенного исследования после однократного и многократного приема фавипиравира можно сделать вывод, что фармакокинетический профиль препарата в целом соответствует представлениям об ожидаемых особенностях его ФК с учетом того, что в анализ были включены пациенты с COVID-19 европеодной расы, которые получали значительное количество препаратов сопутствующей терапии помимо фавипиравира [6].
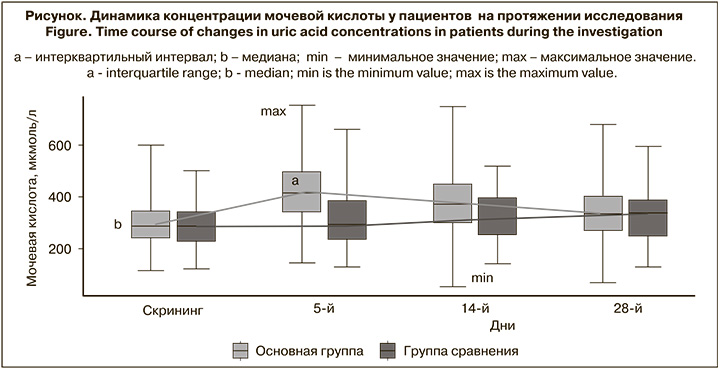
В анализ безопасности были включены данные 163 пациентов, получивших хотя бы 1 дозу препарата: фавипиравира (основная группа, п = 108) или умифеновира, гидроксихлорохина (группа сравнения, п = 55) В ходе исследования какие-либо НЯ были зарегистрированы у 80 (74,1%) пациентов в основной группе и у 33 (60,0%) – в группе сравнения. Разница не была статистически значимой (р = 0,074) и объясняется большим числом случаев гиперурикемии в основной группе (у 41,7% vs 3,6%; р <0,001), что соответствует данным литературы [7]. Большинство НЯ имели легкую степень тяжести. В общей сложности было зарегистрировано 12 тяжелых НЯ. В основной группе отмечено одновременное повышение уровней АЛТ и АСТ у 1 пациента, 1 случай изолированного повышения АСТ и 2 случая повышения АЛТ. В группе сравнения было зарегистрировано 4 НЯ 3-й степени: повышение уровней КФК – у 1 пациента, АЛТ – у 1 пациента, одновременно АСТ и АЛТ – у 1 пациента. Статистически значимых различий между группами по частоте тяжелых НЯ не наблюдалось. Во всех случаях значимого повышения показателей АСТ и/или АЛТ пациенты, помимо фавипиравира, получали значительное количество препаратов сопутствующей терапии, включая те, которые сами по себе способны вызвать повышение уровней печеночных трансаминаз (например, азитромицин, левофлоксацин, амоксициллин/сульбактам, цефтриаксон и ибупрофен).
Отмена препарата в связи с НЯ потребовалась 2 пациентам в основной группе (в связи с повышением показателей АЛТ и АСТ 3-й и 2-й степени) и 1 пациенту в группе сравнения, получавшему гидроксихлорохин, в связи с повышением АЛТ 3-й степени. После отмены терапии НЯ разрешились без негативных последствий. Случаи досрочного выбывания пациентов из исследования из-за НЯ не выявлены. Было зарегистрировано 2 случая серьезных НЯ в основной группе: перелом лучевой кости и снижение сатурации кислородом (проявление прогрессирования основного заболевания). Очевидно, что они не были связаны с приемом фавипиравира.
Случаев смерти в исследовании также не зарегистрировано (табл. 1).

Спектр зарегистрированных НЯ в целом соответствует описанному в литературе профилю безопасности фавипиравира [8]. В основной группе более чем у 5% пациентов проявились НЯ со стороны ЖКТ (диарея, тошнота, боль в животе, боль в верхних отделах живота). Более чем у 10% пациентов были зарегистрированы следующие лабораторные отклонения – гиперурикемия, повышение уровней АЛТ АСТ, КФК, гипергликемия (табл. 2).
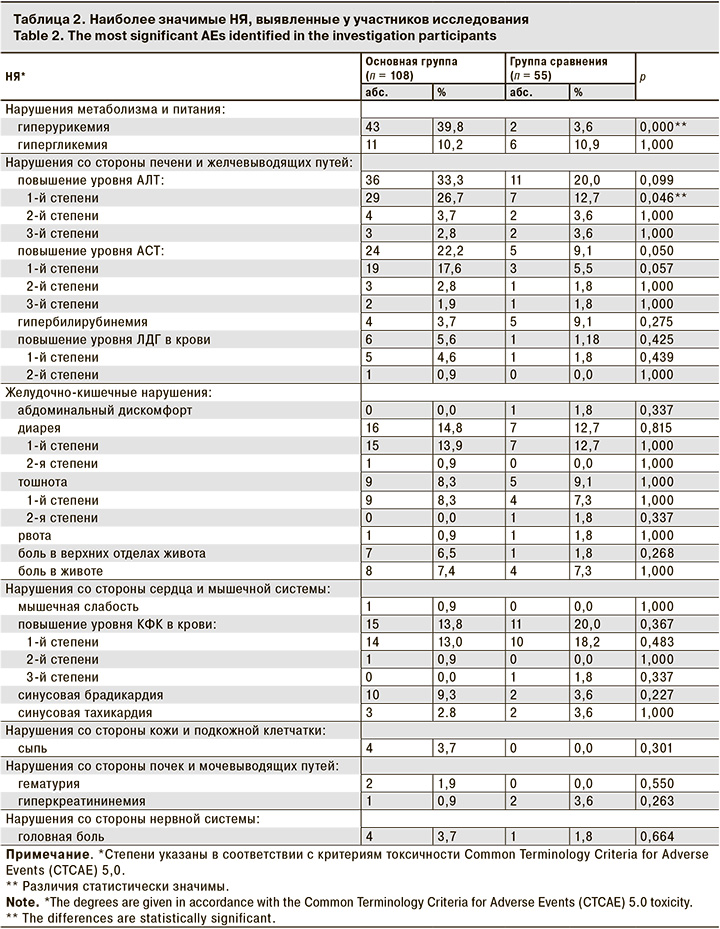
Зарегистрированы достоверные различия по частоте проявления гиперурикемии, которая наблюдались у 45 (41,7%) пациентов основной группы и у 2 (3,6%) пациентов группы сравнения (р < 0,001). Среднее значение в основной группе на 5-й день составило 420,53 мкмоль/л, а в группе сравнения – 306,60 мкмоль/л (р < 0,001). После окончания терапии, на 14-й день от начала лечения, различия между группами были менее значимы (376,01 мкмоль/л vs 326,8 сответственно; р < 0,05), а на 28-й день различия не отмечены [9]. Повышение уровня мочевой кислоты в крови – частый побочный эффект фавипиравира. Препарат метаболизируется до неактивного метаболита M1 альдегидоксидазой и ксантиноксидазой и выводится с мочой. В почках выведение мочевой кислоты регулируется балансом реабсорбции и канальцевой секреции в проксимальных канальцах. Фавипиравир и M1 действуют как умеренные ингибиторы переносчиков органических анионов 1 и 3 (OAT1 и OAT3), которые участвуют в экскреции мочевой кислоты в почках. Кроме того, M1 усиливает обратный захват мочевой кислоты через транспортер уратов 1 (URAT1) в проксимальных канальцах почек. Таким образом, фавипиравир снижает выведение мочевой кислоты с мочой, что приводит к повышению ее концентрации в крови [10]. После завершения терапии все случаи гиперурикемии разрешились, что также согласуется с литературными данными. Следует отметить, что медиана уровня мочевой кислоты не выходит за пределы референтных значений, при этом в динамике отмечается тенденция к восстановлению показателя (см. рисунок).
Согласно протоколу, в подавляющем большинстве случаев исследователи регистрировали в качестве НЯ даже минимальные отклонения уровня мочевой кислоты от нормы, поэтому полученные нами результаты могут отличаться от представленных в литературе данных.
В основной группе чаще отмечали случаи повышения уровней «печеночных» трансаминаз, которые, по мнению исследователей, были связаны с приемом препарата: АЛТ – у 36 (33,3%) пациентов в основной группе и у 11 (20%) – в группе сравнения, АСТ – у 24 (22,2%) и 5 (9,1%) соответственно, однако, статистически значимой разницы между средними значениями уровней трансаминаз не выявлено. Проявление данного НЯ на фоне терапии фавипиравиром является ожидаемым и носит транзиторный характер, но в то же время может быть связано и с другими препаратами.
Обсуждение
Назначение этиотропной терапии необходимо при COVID-19, как и при любой другой вирусной инфекции. Это обеспечивает более ранний выход из болезни и способствует снижению количества осложнений в период реконвалесценции.
Благодаря механизму прямого противовирусного действия препарат фавипиравира «КОРОНАВИР» демонстрирует высокую эффективность в лечении пациентов с COVID-19. По результатам оценки ФК можно предположить наличие кумуляции препарата при многократном применении. В целом его фармакокинетические показатели соответствуют ожидаемым для фавипиравира у пациентов европеодной расы. Благоприятный профиль безопасности характеризуется крайне незначительной выраженностью симптоматических реакций при приеме препарата, субъективно пациенты благополучно переносят терапию. Более выражены были отклонения биохимических показателей крови, в подавляющем большинстве случаев – легкой степени, у всех пациентов они носили временный характер. Возможно, часть зарегистрированных отклонений связана с применением не только и не столько противовирусных средств, сколько с сочетанной терапией, включающей прием нестероидных противовоспалительных и антибактериальных препаратов, иной сопутствующей медикаментозной коррекции, влияние которых на существенное изменение лабораторных показателей общеизвестно.
Выводы
1. Применение этиотропной терапии при лечении COVID-19 на ранних этапах и при нетяжелых формах заболевания оправданно, необходимо и способствует снижению риска развития осложнений.
2. Выбор фавипиравира в качестве стартового препарата обоснован достаточно ранней элиминацией вируса SARS-CoV-2 и значительно более ранним (на 4 дня) наступлением клинического улучшения по шкале ВОЗ, чем при использовании умифеновира и гидроксихлорохина.
3. При оценке профиля безопасности фавипиравира не было отмечено серьезных НЯ, связанных с его приемом. Однако при назначении препарата следует помнить, что он противопоказан к применению у беременных в связи с тератогенным влиянием на плод. Кроме того, его применение требует дополнительной контрацепции как для мужчин, так и для женщин на всем протяжении приема и в течение 3 мес. после него.


