В настоящее время острые респираторные вирусные инфекции (ОРВИ), включая грипп, представляют собой одну из самых актуальных медицинских и социально-экономических проблем. ОРВИ прочно занимают основное место в структуре инфекционной заболеваемости во всем мире. Так, по данным ВОЗ, в мире в среднем около 1 млрд человек в год заболевают гриппом, при этом у 3–5 млн из них развиваются тяжелые формы инфекции, а от 300 000 до 500 000 больных умирают [1, 2]. Годовой экономический ущерб от ОРВИ составляет более 77% общего ущерба от инфекционных болезней. В России ущерб от инфекционных заболеваний составляет около 100 млрд руб. в год, при этом на грипп и ОРВИ приходится до 90% от всей регистрируемой инфекционной патологии [3, 4].
ВОЗ подчеркивает, что противовирусные препараты приобретают особое значение как единственное специфическое медикаментозное средство снижения заболеваемости и смертности от гриппа. В настоящий момент базовыми препаратами для лечения гриппа, рекомендованными ВОЗ, являются химиопрепараты этиотропного действия (осельтамивир и занамивир), оказывающие непосредственное прямое воздействие на размножение вируса путем ингибирования поверхностного гликопротеина вирусов гриппа А и В.
Основными условиями эффективного лечения гриппа являются раннее начало приема этиотропных препаратов с учетом резистентности циркулирующих штаммов вируса, проведение противовоспалительной и дезинтоксикационной терапии. Всем пациентам, включая беременных, пожилых и пациентов с сопутствующими заболеваниями, противовирусные препараты рекомендуется назначать как можно раньше, с проявлением первых клинических признаков заболевания, и не позднее 48 ч. При развитии тяжелых форм противовирусные препараты назначают с момента поступления пациента в стационар, независимо от дня болезни [3, 5, 6].
В России в течение многих лет для лечения и профилактики гриппа широко и успешно используются интерфероны, индукторы интерферона, иммуномодуляторы [3, 7, 8]. Широкое распространение получил отечественный препарат арбидол (умифеновир), обладающий более широким спектром действия и эффективный в отношении вирусов гриппа и возбудителей ОРВИ. Приказом Минздрава СССР № 229 от 23 марта 1988 г. он разрешен для медицинского применения у взрослых в качестве лечебного средства при гриппе, а для лечения и профилактики гриппа и ОРВИ у детей – с 1995 г. Препарат успешно применяется на территории Российской Федерации для лечения гриппа в течение более 15 лет. Он малотоксичен, эффективен и доступен по стоимости [8–10].
Многочисленные экспериментальные и клинические исследования показали, что эффективность умифеновира является результатом широкого спектра его биологической активности и обусловлена непосредственно вирусспецифическим действием: он действует на ранних стадиях вирусной репродукции и ингибирует слияние вирусной липидной оболочки с внутриклеточными мембранами, предотвращая проникновение вируса внутрь клетки, но не влияет на вирусную транскрипцию и трансляцию, а также на активность нейраминидазы и адсорбцию вируса [11–13].
Целью настоящего исследования явилась оценка клинической эффективности умифеновира при ОРВИ у взрослых пациентов.
 В эпидемическом сезоне 2016–2017 гг. проводилось амбулаторное лечение лиц в возрасте от 18 до 60 лет, обратившихся за медицинской помощью с жалобами на лихорадку, озноб, слабость, кашель, насморк, першение в горле, чихание.
В эпидемическом сезоне 2016–2017 гг. проводилось амбулаторное лечение лиц в возрасте от 18 до 60 лет, обратившихся за медицинской помощью с жалобами на лихорадку, озноб, слабость, кашель, насморк, першение в горле, чихание.
Для верификации диагноза проведена экспресс-диагностика гриппа помощью иммунохроматографического теста с использованием тест-системы Influenza A+B («Vegal Farmaceutica S.L.», Испания), ПЦР на вирусы гриппа и ОРВИ (взяты мазки из носоглотки). Всем пациентам проведено комплексное обследование, включающее клинический анализ крови, общий анализ мочи, рентгенографию органов грудной клетки и электрокардиографию (по необходимости). Определены в динамике (в 1-й и на 7-й дни наблюдения) показатели фагоцитарной активности моноцитов, гранулоцитов и «интерфероновый статус» по методике С.С. Григорьян и соавт. [14]. Статистическую обработку данных осуществляли с помощью программы Statistica 8.0 («StatSoft Inc.», США) в соответствии с общепринятыми методами биомедицинской статистики. Сравнение независимых выборок по количественным характеристикам проводили с помощью U-критерия Манна–Уитни, зависимых выборок (показателей до и после лечения) – c помощью Т-критерия Вилкоксона. При сравнении независимых выборок по качественным признакам использовали критерий χ2 с поправкой Йетса. Нулевая гипотеза отвергалась при p < 0,05.
Материалы и методы
Под наблюдением находились 47 больных ОРВИ в возрасте от 18 до 60 лет, из них 26 (55,3%) мужчин и 21 (44,7%) женщина. 53,2% составили лица в возрасте от 18 до 30 лет. Критериями исключения были прием противовирусных и системно действующих иммуномодулирующих препаратов в течение 1 мес. до госпитализации, тяжелое течение ОРВИ, наличие осложнений заболевания в момент обращения или обострение сопутствующих заболеваний на фоне ОРВИ.
Пациенты были разделены на 2 группы в зависимости от сроков обращения и начала терапии. В 1-ю группу (n = 23) вошли пациенты, которые начали получать лечение в первые 48 ч после появления симптомов заболевания, во 2-ю (n = 24) – пациенты, обратившиеся за медицинской помощью на 2–3-и сутки болезни. У всех больных ОРВИ протекала в легкой или среднетяжелой форме. Всем пациентам был назначен умифеновир по 2 капсулы 4 раза в день в течение 5 дней на фоне сопутствующей симптоматической терапии.
Осмотр пациентов проводили в день обращения, на 4-й день лечения и на 6–7-й день – после завершения противовирусной терапии. Во время осмотра регистрировали клинические симптомы заболевания, оценивал интенсивность их проявления и переносимость препарата. Пациентам 2 раза в день проводили термометрию. Эффективность терапии оценивали в зависимости от сроков нормализации температуры тела, исчезновения проявлений интоксикации (головная боль, слабость, головокружение), продолжительности катаральных симптомов (ринит, заложенность носа, кашель, «першение» и боль в горле).
Методом ПЦР у больных выявляли тип возбудителя ОРВИ: вирус гриппа типа А был обнаружен у 25,5% пациентов, вирус гриппа типа В – у 10,6%, риновирус – у 8,6%, аденовирус – у 6,4%, вирус парагриппа типа 1 – у 2,1%, респираторно-синцитиальный вирус – у 4,3%, вирусно-бактериальные ассоциации (вирусы гриппа или парагриппа в сочетании с Streptococcus pneumoniae или Haemophilus influenzae) – у 10,6%, бактериальная флора (Streptococcus pneumonia, Staphylococcus aureus) – у 8,5%. В 23,5% случаев не удалось обнаружить возбудителей респираторных инфекций.
Результаты
У всех пациентов заболевание начиналось остро, у 8,5% из них температура тела не превышала 37 °С, у 31,9% – 37–37,9 °С, у 51,1% – 38–38,9 °С и у 8,5% поднималась выше 39 °С. Жалобы на общую слабость предъявляли 91,5% пациентов, на озноб – 44,7%. Кашель наблюдался у 66% больных, в 45% случаев – сухой.
В общем анализе крови лейкоцитоз регистрировали в 23,4% случаев, лейкопению – в 36,2%. При изучении динамики показателей клинического и биохимического анализов крови, общего анализа мочи статистически значимых различий между группами не выявлено.
Эффективность терапии оценивали по длительности сохранения симптомов ОРВИ (см. таблицу). Клинический эффект отмечен в отношении катарально-респираторного синдрома и сокращения лихорадочного периода, более быстрого восстановления пациентов 1-й группы. Длительность лихорадки у больных в 1-й группе в среднем составляла 3,76 ± 0,17, во 2-й – 4,9 ± 0,2.
Оценка эффективности умифеновира при ОРВИ у невакцинированных людей в возрасте 18–60 лет без сопутствующих заболеваний в стадии обострения показала достоверное снижение проявлений интоксикационного синдрома с максимальным клиническим эффектом у 76% больных 1-й группы уже на 2-е сутки приема препарата. В 1-й группе средняя продолжительность заболевания составила 4,1 ± 0,2 дня, во 2-й – 6,9 ± 0,38. Осложнения развились у 6 пациентов: в 1-й группе у 1, во 2-й – у 5. Антибактериальная терапия была назначена на 4-й день 5 из них; 1 пациент из 2-й группы в возрасте 57 лет госпитализирован с осложнением на фоне гриппа А (пневмония) в инфекционный стационар (в анамнезе – сахарный диабет II типа).
 Несомненно, клинический эффект препарата напрямую связан с подавлением вирусной агрессии, уменьшением уровня провоспалительных цитокинов, снижением адгезивной способности мононуклеарных клеток крови к активации лимфоцитов, стимуляции фагоцитарной системы макрофагов и нейтрофилов, росту и активации NK-клеток [8, 10, 15, 16]. Такие сдвиги в иммунной системе способствуют подавлению бактериальной и грибковой флоры, определяя неосложненное течение заболевания.
Несомненно, клинический эффект препарата напрямую связан с подавлением вирусной агрессии, уменьшением уровня провоспалительных цитокинов, снижением адгезивной способности мононуклеарных клеток крови к активации лимфоцитов, стимуляции фагоцитарной системы макрофагов и нейтрофилов, росту и активации NK-клеток [8, 10, 15, 16]. Такие сдвиги в иммунной системе способствуют подавлению бактериальной и грибковой флоры, определяя неосложненное течение заболевания.
Обсуждение
Полученные нами результаты согласуются с данными клинических исследований, в которых показано, что умифеновир сокращает общую продолжительность ОРВИ в среднем на 47%. Убедительно доказано, что назначение препарата пациентам в ранние сроки позволяет не только существенно сократить продолжительность заболевания, но и уменьшить тяжесть его проявлений и осложнений [3, 8, 15]. Было установлено, что применение умифеновира в первые 48 ч от начала заболевания многократно снижало частоту развития осложнений со стороны дыхательных путей.
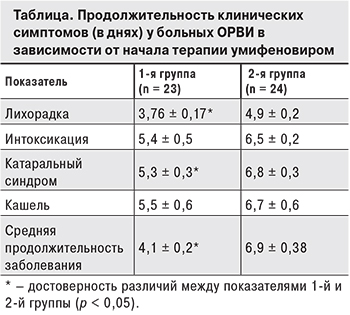
Заслуживают внимания изменения в интерфероновой системе. Исходный уровень ИФН-подобной противовирусной активности сыворотки крови был в пределах референсных значений (2–8 усл. ед.), принятых за норму (рис. 1). Полученные результаты согласуются с заключением М.В. Шипилова [16] о том, что изменения в интерфероной системе не являются определяющими при ОРВИ.
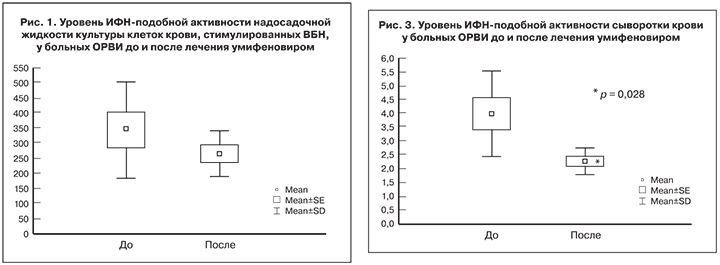
Изучение других показателей, характеризующих ИФН-подобную активность супернатантов культур клеток крови, инкубированных в среде с добавлением ВБН (индуктора ИФН I типа) или без него, не выявило статистически значимых различий между показателями пациентов 1-й и 2-й группы ни до, ни после проведенного лечения. При этом средняя ВБН-индуцированная продукция ИФН-подобных факторов была примерно в 2 раза меньше нижней границы диапазона условной нормы (640–1280 усл. ед.), а спонтанная выработка этих факторов – на уровне референсных значений (< 2 усл. ед.) (рис. 2).
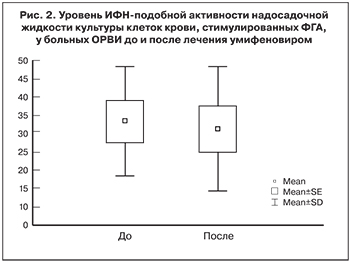
Тяжесть ОРВИ во многих случаях определяется не только прямым или опосредованным патогенным действием вирусов, но и выраженностью вторичных бактериальных осложнений. При развитии бактериальной суперинфекции ИФН I типа играют неоднозначную роль в иммуном ответе [3, 8, 12]. Авторы указанных исследований подтвердили стимулирующее влияние умифеновира на неспецифический иммунитет и фагоцитоз: прием препарата достоверно увеличивал фагоцитарный индекс с 3,0 ± 1,1 до 5,9 ± 1,7% после лечения, а также фагоцитарное число – с 3,1 ± 1,2 до 7,3 ± 1,5 в динамике терапии. Наблюдается выраженная стимуляция сыворочного ИФН в динамике терапии умифеновиром (рис. 3).
Основным клиническим эффектом этиотропного противовирусного препарата является защита от развития вторичных бактериальных осложнений и улучшение показателей неспецифического иммунитета при назначении препарата в первые 48 ч. Наши данные согласуются с промежуточными результатами многоцентрового двойного слепого рандомизированного плацебо-контролируемого исследования АРБИТР [17], в котором у взрослых пациентов в возрасте от 18 до 70 лет с симптомами ОРВИ и гриппа рано начатое лечение умифеновиром ассоциировалось с достоверно легким течением заболевания (р = 0,01) и быстрым выздоровлением по сравнению с пациентами, принимавшими плацебо.
Заключение
Таким образом, в проведенном исследовании было установлено, что прием умифеновира в первые 48 ч после начала заболевания позволяет значительно сократить продолжительность болезни и длительность проявления основных ее симптомов, уменьшить тяжесть течения и снизить риск развития осложнений.


