Папилломавирусная инфекция является одной из самых распространенных в мире среди инфекций, передаваемых половым путем. На сегодняшний день известно более 200 типов вируса папилломы человека (ВПЧ) [1].
ВПЧ является основным этиологическим агентом рака шейки матки (РШМ) благодаря своей способности инициировать различные пролиферативные процессы в клетках плоского и железистого эпителия, в особенности в области зоны трансформации. Этот факт неоднократно подтвержден многочисленными молекулярно-биологическими исследованиями и эпидемиологическими данными. В целом к ВПЧ-ассоциированным ракам относят до 99,7% всех случаев РШМ [2–4], оставшиеся по различным данным 0,3–0,7% истинных ВПЧ-отрицательных случаев злокачественной патологии вызваны генетически-обусловленными либо иными факторами [3, 4].
Основываясь на эпидемиологических и биологических данных, в 2012 г. ВОЗ предложила разделение ВПЧ на группы по их трансформирующей активности [5].
К группе 1 отнесены 12 типов ВПЧ (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59), они определены как типы, канцерогенные для человека (carcinogenic to humans) и выявляются примерно в 96,5% всех случаев РШМ в мире. При этом около 70% случаев PШМ связаны с ВПЧ 16 и 18 типов и еще около 20% – с типами 31, 33, 35, 45, 52 и 58 [4]. Канцерогенные типы ВПЧ могут являться причиной рака влагалища, вульвы, полового члена, анального канала/прямой кишки и ротоглотки, а также, по некоторым данным, немелкоклеточного рака легких [6, 7].
К группе 2А – вероятно канцерогенных для человека (probably carcinogenic to humans) – отнесен 68 тип ВПЧ. Группу 2В – возможно канцерогенных для человека (possibly carcinogenic to humans) – составляют 7 типов ВПЧ: 26, 53, 66, 67, 70, 73, 82. Они менее распространены в популяции, однако могут являться единственной этиологической причиной ВПЧ-ассоциированного РШМ (≤ 1% на каждый) [8]. Еще 5 типов ВПЧ (30, 34, 69, 85, 97) отнесены к возможно канцерогенным для человека (possibly carcinogenic to humans) на основании их филогенетического родства с типами с достаточным или ограниченным доказательством канцерогенности для человека [4]. Поскольку в научной литературе продолжается широкое использование принятых ранее названий, а состав типов по группам не содержит противоречий, здесь и далее типы, входящие в группу 1, будут именоваться «ВПЧ высокого канцерогенного риска» (ВПЧ ВКР), группу 2А – «ВПЧ вероятно высокого канцерогенного риска» (ВПЧ вероятно ВКР), группу 2В – «ВПЧ возможно высокого канцерогенного риска» (ВПЧ возможно ВКР).
Суммарная распространенность 8 наиболее часто встречающихся типов ВПЧ вероятно и возможно ВКР (26, 53, 66, 67, 68, 70, 73, 82) при РШМ составляет около 3% [4, 8]. Это означает, что данные типы ВПЧ могут ежегодно вызывать около 15 000 новых случаев РШМ и 7000 смертей [9].
Общеизвестными являются сведения о различии в распространенности разных типов ВПЧ в разнообразных этникогеографических областях. Так, в среднем частота встречаемости в зависимости от региона, в котором проводилась выборка, варьирует от 5 до 41,6%, и коррелирует с социально-экономическими, поведенческими, медицинскими и другими стандартами данной территории [10, 11].
Цель исследования – изучение распространенности ВПЧ возможно ВКР и его вклада в развитие злокачественных новообразований шейки матки с оценкой достоверности получаемых данных в зависимости от используемых диагностических подходов.
Материалы и методы
Поиск выполняли в базах данных MEDLINE (PubMed), «КиберЛенинка» и eLibrary, по ключевым словам, на русском и английском языках: ВПЧ, ВПЧ возможно ВКР, РШМ, ВПЧ-отрицательный РШМ, ВПЧ 26 типа, ВПЧ 53 типа, ВПЧ 66 типа, ВПЧ 67 типа, ВПЧ 70 типа, ВПЧ 73 типа, ВПЧ 82 типа. В ходе работы проанализировано более 400 статей на русском, английском и немецком языках (глубина поиска – 21 год). Критериями исключения работ из анализа были отсутствие указания точного количества выявленных типов ВПЧ; проведение ВПЧ-тестирования с помощью наборов реагентов, не дифференцирующих ВПЧ по типам или дифференцирующих ограниченное количество типов; отсутствие информации о количестве полученных отрицательных образцов в исследовании.
Проанализированы представленные авторами данные по распространенности ВПЧ различного канцерогенного риска, вкладу 25 типов ВПЧ (суммарно и по отдельности) в развитие злокачественной патологии шейки матки.
Статистическую неоднородность исследований оценивали с помощью индекса гетерогенности (I2) и Q-критерия Кохрена (Cochran’s Q test). При этом анализируемые группы считали гетерогенными при I2 > 50% и p-значения для Q-критерия Кохрена < 0,1. При высокой гетерогенности была использована модель случайных эффектов, при низкой – фиксированных [12]. Исследования, вносящие гетерогенность в общий эффект, были дополнительно оценены с помощью расстояния Кука и стандартизованного отклонения от суммарного эффекта [13]. Статистическая обработка результатов и визуализация данных выполнены в среде R [14].
Результаты
В метаанализ включены 16 работ, выполненных в 2001–2022 гг. исследователями из Российской Федерации, Республики Беларусь и Азербайджанской Республики, Соединенных Штатов Америки, Бразилии, Италии, Испании, Нидерландов, Португалии, Австрии, Китайской Народной Республики, Японии, Монголии, Израиля.
Суммарная выборка из проанализированных публикаций – 30 090 женщин в возрасте от 12 до 89 лет – была разделена на 3 группы. 1-ю группу составили 20 989 условно-здоровых пациенток с цитологическим заключением NILM (negative for intraepithelial lesion or malignancy – отсутствие интраэпителиальных поражений). Во 2-ю группу были включены 2785 пациенток с цитологическим заключением LSIL (low-grade squamous intraepithelial lesions – плоскоклеточное интраэпителиальное поражение низкой степени) и ASCUS (atypical squamous cells of undetermined significance – клетки плоского эпителия неясного значения). 3-ю группу составили 6316 женщин с цитологическим заключением HSIL/Carcinoma (HSIL – high-grade squamous intraepithelial lesions – плоскоклеточное интраэпителиальное поражение высокой степени), объединяющим CIN II (умеренная цервикальная интраэпителиальная неоплазия), CIN III (тяжелая цервикальная интраэпителиальная неоплазия) и РШМ.
Анализ исследований, вносящих гетерогенность, показал, что минимальное количество публикаций имело наибольший эффект на общий результат и при этом отклонялось от суммарного эффекта. Причины такого отклонения не выходили за пределы разного объема выборки и этногеографических различий по распространенности типов ВПЧ. Чтобы избежать влияния этого типа исследований на суммарный эффект, использована модель случайных эффектов с более низким весом данных публикаций и большим доверительным интервалом (ДИ).
По данным метаанализа, у 79% (95% ДИ 44–95) пациенток 1-й группы ВПЧ не обнаружен. Во 2-й группе ВПЧ-отрицательными оказались 30% (95% ДИ 4–80) обследованных, что в 2,6 раза меньше, чем в 1-й группе (рис. 1). При этом гетерогенность публикаций (I2 > 50%; p < 0,1) и использование модели случайных эффектов с большим ДИ не позволяет с точностью сказать о статистически достоверных различиях между выборками.

Доля ВПЧ-положительных результатов в 1-й группе составила 20% (95% ДИ 5–56), среди них ВПЧ ВКР – 70% (95% ДИ 62–77), ВПЧ возможно ВКР – 14% (95% ДИ 9–20), на все остальные типы пришлось 11% (95% ДИ 1–64). При анализе представленных данных случаи выявления сочетанного инфицирования ВПЧ разных типов отдельно не учитывали, так как они были описаны единично [10, 15, 16].
В 1-й группе среди ВПЧ ВКР были наиболее распространены типы 52 (12%), 16 (9%) и 51 (7%). Среди ВПЧ возможно ВКР первое ранговое место занял 53 тип (6%), второе – 68 и 66 типы (по 3% каждый) (табл. 1). Во 2-й группе положительные результаты составили 70% (95% ДИ 19–96), из них ВПЧ ВКР – 71% (95% ДИ 40–87), 7 типов ВПЧ возможно ВКР – 20% (95% ДИ 13–29), остальные 27 типов – 13% (95% ДИ 5–28), включая моно- и сочетанные выявления (см. рис. 1).

Отмечены различия по частоте встречаемости разных типов ВПЧ между 1-й и 2-й группами. Так, в 1-й группе среди ВПЧ ВКР 52 тип занял 1-е, а 16 тип – только 2-е ранговое место. Во 2-й группе самым распространенным являлся 16 тип (14%), далее следовали типы 31, 52 и 56 (по 6% соответственно). Среди ВПЧ возможно ВКР во 2-й группе 1-е место занял 66 тип (8%), 53 тип встречался в 7% случаев (табл. 2).

При анализе данных, представленных разными исследователями, нельзя не обратить внимания на достаточно частое упоминание о случаях ВПЧ-отрицательного РШМ. Они обусловлены различными диагностическими подходами, а именно конструкторскими особенностями наборов реагентов, применяемых при тестировании образцов. При анализе ВПЧ-ассоциированного РШМ с использованием наборов реагентов, позволяющих детектировать только ВПЧ ВКР, нерасшифрованными остаются все случаи РШМ, вызванные ВПЧ вероятно ВКР, возможно ВКР и др. Существует ряд наборов реагентов, где в качестве диагностической мишени выбран L1 ген ВПЧ. Такой подход позволяет идентифицировать все известные типы ВПЧ, за исключением интегрированных в геном человека, что является существенным ограничением теста и приводит к появлению ложноотрицательных результатов, ошибочно интерпретируемым как ВПЧ-отрицательные случаи РШМ. Показано, что в процессе интеграции ВПЧ в геном клетки-хозяина происходит в большей мере потеря гена L1, кроме того существуют доказательства прямой взаимосвязи процесса интеграции с прогрессированием неопластического процесса [17, 18]. При анализе тех же образцов с применением наборов реагентов, где используются для выявления типоспецифичные праймеры, нацеленные на область E6–E7 гена вируса, возбудитель идентифицируется. Соответственно наборы реагентов, в которых диагностической мишенью является область L1 генома вируса, использовать при анализе образцов пациенток с злокачественными изменениями тканей шейки матки необоснованно [19].
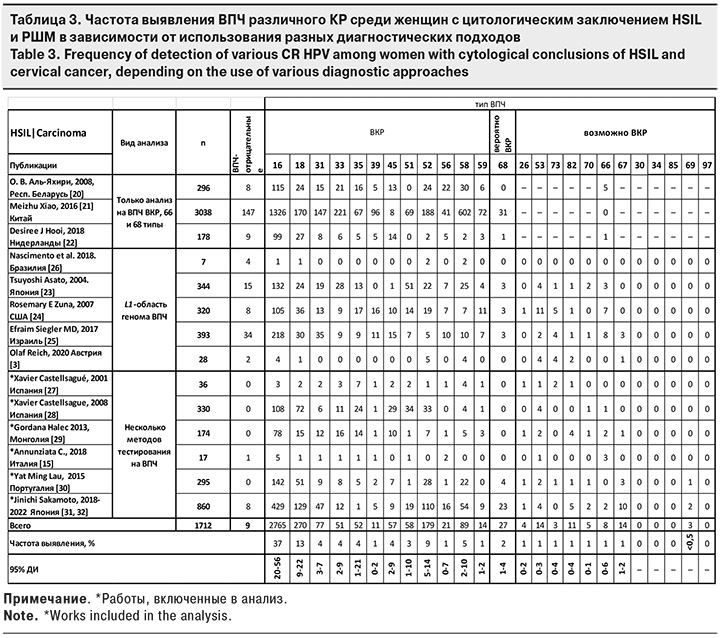
Работы, вошедшие в 3-ю группу (HSIL/Carcinoma), дополнительно были разделены на 3 подгруппы (табл. 3). В подгруппу 1 (n = 3512) вошли публикации, в которых представлены данные о проведении тестирования исследуемого материала при помощи только одного набора реагентов, позволяющего идентифицировать ВПЧ ВКР и 66, 68 типы по участку генов E6 и Е7 [20–22]. В подгруппе 2 (n = 1092) образцы тестировали с помощью наборов реагентов, позволяющих осуществлять типирование ВПЧ по консервативному участку гена L1, кодирующему капсидный белок вируса [3, 23–26]. В подгруппе 3 (n = 1712) образцы подвергали многоэтапному исследованию, направленному на этиологическую верификацию – определение ВПЧ известных типов различной канцерогенной активности с использованием наборов реагентов, типирующих ВПЧ по участку гена L1, онкогенам E6 и Е7, секвенирования и др., а образцы, оказавшиеся истинно ВПЧ-отрицательными, дополнительно изучали с целью выявления известных онкомутаций человека [15, 27–32].
Метаанализ полученных результатов показал, что в подгруппе 1 ВПЧ-отрицательными остались 5% (95% ДИ 4–5) образцов HSIL/Carcinoma, в подгруппе 2 – 8% (95% ДИ 2–36), а подгруппе 3 с углубленным изучением – всего 1% (95% ДИ 0–2) (рис. 2). Различия оказались статистически значимыми между подгруппами 1 и 3, а также 2 и 3. ДИ у подгрупп 1 и 3, 2 и 3 не перекрывались, что может косвенно подтвердить статистически значимую разницу между выборками.

Таким образом, большой процент ВПЧ-отрицательных образцов среди HSIL/Carcinoma может косвенно свидетельствовать о несовершенстве использованных методик тестирования биологического материала.
Исходя из вышесказанного, частота выявления типов ВПЧ ВКР и возможно ВКР среди пациенток с цитологическим заключением HSIL и РШМ в представляемом метаанализе определена на основании работ X. Castellsagué и соавт. [27, 28], G. Halec и соавт. [29], Y.M. Lau и соавт. [30], C. Annunziata и соавт. [15], J. Sakamoto и соавт. [31, 32].
На долю ВПЧ ВКР приходится более 85%, ВПЧ вероятно/возможно ВКР 26, 53, 66, 67, 68, 70, 73, 82 типов составляют суммарно 6% (95% ДИ 3–16), 5 типов ВПЧ вероятно КР (30, 34, 69, 85, 97) – менее 0,5% (табл. 3). 21 тип неизвестного КР (12, 32, 40, 42, 43, 50, 54, 55, 61, 62, 71, 72, 74, 81, 83, 84, 86, 89, 90, 91, 108) составили суммарно менее 0,5%.
Большинство авторов не предоставили информацию о моно- или сочетанном инфицировании ВПЧ возможно ВКР. По этой причине в ряде случаев невозможно было оценить, являлись ли данные типы этиологическим агентом развития РШМ. Однако ряд работ либо содержал упоминания об обнаруженных случаях моноинфицирования ВПЧ возможно ВКР при РШМ, либо был нацелен на поиск и изучение подобных редких случаев.
Так, в исследовании M. Ciotti с соавт. [33], проведенном в Италии, среди 102 случаев заболевания РШМ выявлен только 1 случай моноинфицирования ВПЧ возможно ВКР 73 типа.
В совместной работе ученых из Нидерландов и Испании под руководством D. Geraets [34] изучены образцы тканей инвазивного РШМ для поиска случаев моноинфицирования редкими типами ВПЧ. При проведении ретроспективных изысканий исследовано 10 575 архивных парафинированных образцов тканей за период с 1949 по 2009 г. После нескольких этапов тестирования, включающих проведение секвенирования, выявлен 121 образец с редко встречающимися типами ВПЧ: 31 образец содержал ВПЧ 26 типа и столько же образцов – ВПЧ 30 типа; в 26 случаях обнаружили 67 тип, в 7 – 69, в 6 – 82, в 3 случаях – 34 тип, в 2 образцах – 68 тип и по 1 случаю пришлось на 61 и 91 типы. Оставшиеся 13 образцов тканевого материала шейки матки содержали либо редкие генетические вариации ВПЧ ВКР 16, 56 и 39 типов, либо типы, которые так и не удалось идентифицировать. Позднее группа ученых из Китая опубликовала результаты изучения прогностического значения выявляемого типа ВПЧ у больных РШМ, получающих первичное лечение [30]. В процессе исследования 236 образцов тканевого материала с гистологически подтвержденным РШМ выявлены несколько случаев моноинфицирования ВПЧ возможно ВКР, где 53 тип обнаружен в 2 образцах, 26 и 70 типы – каждый в 1 образце.
Обширную работу по изучению этиологической причины развития РШМ провела группа японских ученых под руководством J. Sakamoto [31, 32], в ходе которой протестировано 1526 образцов CIN и 371 образец тканей инвазивного РШМ. Установлено 22 случая моноинфицирования 68 типом, 4 – 53 типом, 4 – 82 типом, по 2 случая – 66 и 70 типами и 1 – 26 типом ВПЧ.
Для принятия решения о необходимости включения ВПЧ возможно ВКР в скрининговые программы и эпидемиологический мониторинг, необходимо установить их роль в развитии предраковых заболеваний и РШМ. Тот факт, что типы ВПЧ возможно ВКР встречаются в виде единственного этиологического агента при РШМ, может быть косвенным доказательством его онкоактивности.
Ранее, в процессе исследования наиболее распространенного и обладающего высоким онкогенным потенциалом ВПЧ 16 типа, а в дальнейшем и для остальных типов ВПЧ ВКР, была установлена взаимосвязь канцерогенеза в опухолевой клетке с активностью Е6 и Е7 генов ВПЧ, которые связывают регуляторные белки клеточного цикла (pRb) и апоптотического пути (p53) [35, 36].
В 2012–2014 гг. группа исследователей из Испании, Германии, Нидерландов и Саудовской Аравии под руководством G. Halec углубленно изучила 851 образец биопсийного тканевого материала шейки матки пациенток с диагнозом РШМ и установленным фактом моноинфицирования ВПЧ [29, 37]. Этиологическим агентом онкологической патологии признаны: 82 тип – в 44 случаях РШМ, 53 и 66 типы – в 2 и 26, 67, 70 типы – по 1. Далее 321 образец тканевого материала анализировали на предмет транскрипционной активности вирусных онкогенов и экспрессии суррогатных белковых маркеров развития онкологического процесса. К суррогатным маркерам относят важные биохимические или лабораторные параметры, которые сигнализируют о патологических процессах, но не позволяют судить об исходе заболевания в целом. Оценка суррогатных маркеров является важным прогностическим фактором, позволяющим выявлять тяжелые угрожающие жизни заболевания на ранних стадиях [29, 38].
Согласно данным, полученным G. Halec с соавт. [37], у ВПЧ ВКР типоспецифическая вирусная транскрипционная активность в образцах тканей РШМ составила 95%. При этом статистически значимой разницы в экспрессии мРНК ВПЧ между группами ВКР и вероятно/возможно ВКР не наблюдалось. Результаты исследования доказали транскрипционную активность ВПЧ ВКР 59 типа и возможно ВКР 53, 67, 68, 73 типов и их способность оказывать трансформирующее влияние на ткани шейки матки при моноинфицировании. Предыдущие аналогичные исследования продемонстрировали онкопрофиль для 11 типов ВПЧ ВКР (обследовано 75 образцов биопсийного тканевого материала при РШМ) [29, 39] и для 4 типов ВПЧ возможно ВКР (26, 66, 70 и 82) в 7 образцах биопсийного тканевого материала при РШМ) [29].
Обсуждение
Проведенный метаанализ показал незначительный вклад ВПЧ возможно ВКР в общую эпидемиологическую ситуацию по распространенности вируса в популяции женщин – 14% (95% ДИ 9–20) от всех ВПЧ-положительных результатов. Однако необходимо указать на выявленные ограничения нашего анализа: отсутствие поправки на географический регион, этническую составляющую и другие особенности выборок, включенных в исследование, такие как возраст обследуемых, разные поведенческие характеристики, включая сексуальную активность. Эти ограничения были связаны с отсутствием необходимого объема информации в публикациях для включения дополнительных критериев в метаанализ.
Кроме того, представленные данные о распространенности ВПЧ возможно ВКР среди условно-здоровых женщин в научной литературе ограничены, высок и уровень гетерогенности между публикациями разных авторов.
Полученные нами результаты еще раз подтверждают общемировые данные о том, что по мере возрастания тяжести интрацервикального поражения увеличивается и удельный вес выявляемых ВПЧ разного канцерогенного риска. Ранние изменения в структуре тканей шейки матки, вызванные персистенцией ВПЧ, признаны показателем прогрессирования процесса диспластических преобразований, приводящих впоследствии к РШМ. У женщин с цитологическим заключением LSIL и ASCUS возможна спонтанная элиминация вируса, свидетельствующая о благоприятном прогнозе для них. Однако частота спонтанной элиминации в этой группе значительно ниже, чем у женщин с цитологическим заключением NILM. Большую роль при проведении эпидемиологического мониторинга играют выбранные диагностические подходы. Из-за конструкторских особенностей, используемых для проведения исследования наборов реагентов, могут оставаться нерасшифрованными случаи инфицирования редкими или интегрированными формами ВПЧ разного канцерогенного риска. Данные факторы могут влиять на реальную картину распространенности разных типов ВПЧ (в том числе и возможно ВКР) в популяции, несколько изменяя статистику в сторону как увеличения, так и уменьшения процентного содержания.
Знание реальной эпидемиологической картины распространенности ВПЧ особенно актуально в свете быстро внедряющейся во многих странах программы вакцинопрофилактики ВПЧ-ассоциированных заболеваний, активной миграции населения, появления данных, свидетельствующих об увеличении частоты выявления менее распространенных и ранее редко встречающихся типов ВПЧ [40].
Заключение
Данные, полученные учеными разных стран, подтверждают, что ВПЧ возможно ВКР [по классификации ВОЗ (2012): возможно канцерогенный для человека, группа 2В (possibly carcinogenic to humans)], обладая трансформирующей активностью, может оказаться единственным прогностическим фактором развития РШМ. Этот факт свидетельствует о необходимости рекомендовать включение ВПЧ возможно ВКР в эпидемиологический мониторинг, скрининговые программы профилактики РШМ, мониторинг женщин с диагностированными диспластическими изменениями в тканях шейки матки. Учитывая миграцию населения, в последние десятилетия требуется проведение новых эпидемиологических исследований по распространенности ВПЧ возможно ВКР в группе условно-здоровых женщин для четкой выработки диагностических критериев. Дальнейшие исследования ВПЧ возможно ВКР должны обязательно включать изучение дополнительных факторов, которые влияют на активацию их трансформирующей активности в инфицированных тканях.


