Парвовирусная инфекция человека широко распространена во всем мире. Первичное инфицирование преимущественно происходит в детском возрасте, однако от 30 до 60% лиц детородного возраста все еще остаются восприимчивыми к ее возбудителю – эритропарвовирусу приматов 1 (В19Р), ранее известному как парвовирус В19 [1]. В Республике Беларусь к 20-летнему возрасту иммунно лишь 55% населения, к 45 годам их доля возрастает до 85%, что свидетельствует об активном вовлечении взрослых в эпидемический процесс парвовирусной инфекции и высоком риске инфицирования женщин в период беременности [2]. По данным литературы, в межэпидемический период уровень сероконверсии среди беременных составляет 1–3% [3], во время подъема заболеваемости он может возрастать до 17–18% [4, 5].
Впервые связь парвовирусной инфекции с развитием неиммунной водянки плода (НИВП) была установлена в 1984 г. В дальнейшем было показано, что заболевание женщины в период беременности может приводить также к спонтанным абортам, преждевременным родам, внутриутробной гибели плода (ВУГП), развитию фетальной анемии, гепатоспленомегалии, кардиомиопатии, тромбоцитопении, гипоальбуминемии, неврологическим нарушениям [6, 7]. Риск развития водянки плода считается наибольшим при инфицировании женщины с 9-й по 20-ю нед. гестации, в дальнейшем он снижается и является минимальным в последние 2 мес. беременности. Практически все случаи парвовирусной водянки плода были выявлены между 16-й и 32-й нед. гестации [8, 9].
В Республике Беларусь диагностика парвовирусной инфекции у пациентов с НИВП была начата в 2012 г. на базе Республиканского научно-практического центра эпидемиологии и микробиологии (Минск). Настоящее исследование посвящено анализу лабораторных критериев и исходов НИВП парвовирусной этиологии.
Материалы и методы
В период с 2012 по 2018 г. проведено лабораторное исследование 51 случая НИВП для подтверждения или исключения парвовирусной инфекции: в 24 случаях были обследованы мать и новорожденный или плод, в 21 – только мать и в 6 – только новорожденный. В 44 случаях основанием для исследования послужило выявление признаков водянки плода при скрининговом или внеочередном УЗИ, в 6 – рождение ребенка с признаками водянки плода или ВУГП. Во всех случаях изоиммунизация по антигенам резус-фактора (иммунная водянка) была исключена до проведения обследования на парвовирусную инфекцию.
Материалом для исследования послужили сыворотка крови матери и новорожденного/плода, пуповинная кровь, околоплодные воды, асцитическая жидкость, материал аутопсии плаценты и внутренних органов плода.
IgM- и IgG-антитела к В19Р в сыворотке крови выявляли методом иммуноферментного анализа с использованием коммерческих наборов производства Institut Virion/Serion GmbH (Германия) в соответствии с протоколом производителя. ДНК выделяли из 200 мкл клинического образца с помощью набора QIAamp DNA Blood Mini Kit (Нидерланды). Амплификацию фрагмента генома В19Р проводили методом ПЦР в режиме реального времени с использованием коммерческих наборов производства ФБУН Центральный НИИ эпидемиологии Роспотребнадзора (Россия) (2012–2015 гг.) и РНПЦ эпидемиологии и микробиологии (Беларусь) (2016–2018 гг.) с качественной оценкой результатов.
Статистическую обработку полученных данных проводили на персональном компьютере с использованием программы STATISTICA 10.0. Для описательной статистики рассчитывали медиану (Ме), 25-й и 75-й квартили (Р25 и Р75). Для анализа количественных переменных использовали критерий Манна–Уитни (U). Статистически значимым считали результат при р < 0,05.
Результаты
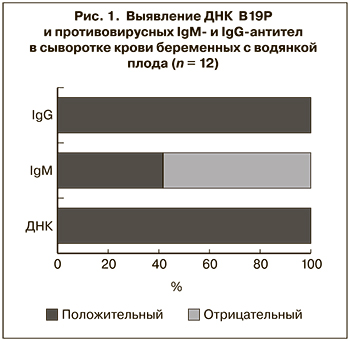 Лабораторная верификация парвовирусной инфекции при НИВП
Лабораторная верификация парвовирусной инфекции при НИВП
В течение 2012–2018 гг. при лабораторном исследовании 51 случая НИВП парвовирусная инфекция была подтверждена в 12 (23,5%) из них. Во всех случаях инфицирование беременных В19Р было заподозрено только после выявления патологии плода, и лабораторное обследование было проведено незамедлительно. Вирусная ДНК была обнаружена в сыворотке крови всех 12 пациенток. Специфические сывороточные IgM-антитела выявлены у 5 (41,7%) из них (рис. 1).
Поскольку ни в одном случае время инфицирования женщины не было достоверно установлено, нельзя исключить, что в 7 случаях, когда IgM отсутствовали, период от инфицирования до обследования был достаточно длительным (более 4 нед.), что и привело к получению отрицательного результата. Подтверждением этого может служить единственный случай, когда время инфицирования можно предполагать с большой долей вероятности (женщина перенесла ОРЗ с артралгиями за 14 нед. до выявления водянки плода), и противовирусные IgM-антитела на момент обнаружения патологии плода у беременной отсутствовали. У всех 12 женщин были выявлены специфические IgG-антитела в концентрации от 20 до 160 МЕ/мл, что подтверждало перенесенную инфекцию.
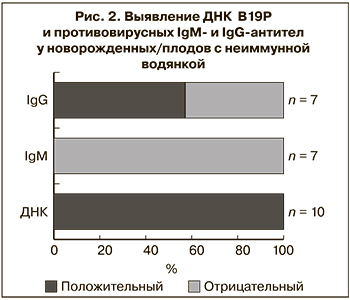 Клинический материал от новорожденных/плодов был предоставлен для исследования в 10 из 12 случаев, в том числе в 7 из них проведено серологическое и ПЦР-исследование, в 3 патологоанатомический материал был исследован только методом ПЦР. Гестационный возраст на момент обследования составил от 20 до 35 нед. ДНК В19Р была обнаружена в клинических образцах всех 10 новорожденных/плодов, в том числе у 6 – в сыворотке крови, у 2 – в околоплодных водах и асцитической жидкости, у 3 – в материале аутопсии плаценты и внутренних органов (рис. 2).
Клинический материал от новорожденных/плодов был предоставлен для исследования в 10 из 12 случаев, в том числе в 7 из них проведено серологическое и ПЦР-исследование, в 3 патологоанатомический материал был исследован только методом ПЦР. Гестационный возраст на момент обследования составил от 20 до 35 нед. ДНК В19Р была обнаружена в клинических образцах всех 10 новорожденных/плодов, в том числе у 6 – в сыворотке крови, у 2 – в околоплодных водах и асцитической жидкости, у 3 – в материале аутопсии плаценты и внутренних органов (рис. 2).
При исследовании сыворотки крови новорожденных IgM-антитела к В19Р не были выявлены ни в одном случае, независимо от срока гестации, на котором проводилось обследование. Противовирусные IgG имели лишь 4 (57,1%) из 7 серологически обследованных новорожденных, несмотря на то что все они родились от IgG-положительных матерей. Хотя наличие у ребенка IgG к В19Р не было связано с их наличием у матери, в 3 из 4 случаев положительный уровень антител имели новорожденные от матерей с наиболее высокой их концентрацией (рис. 3).
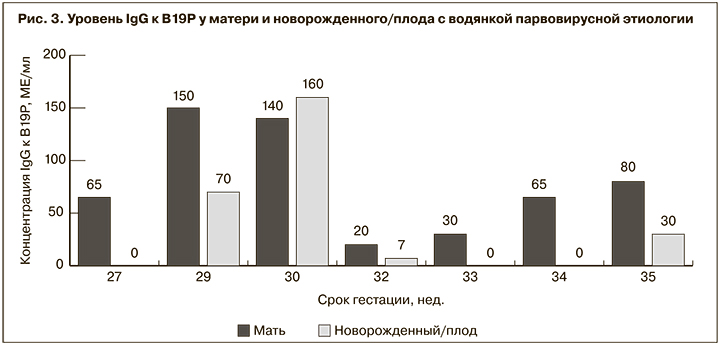
Никакой связи между наличием у новорожденного специфических IgG и сроком гестации выявлено не было: положительный уровень антител выявлен у обследованных в 29, 30, 32 и 35 нед. гестации, их отсутствие – в 27, 33 и 34 нед.
Клинико-эпидемиологическая характеристика случаев НИВП парвовирусной этиологии
 В течение 7 лет наблюдения в Беларуси родилось 786 456 детей, средний многолетний показатель выявления водянки плода парвовирусной этиологии за этот период составил 1,5 на 100 тыс. новорожденных.
В течение 7 лет наблюдения в Беларуси родилось 786 456 детей, средний многолетний показатель выявления водянки плода парвовирусной этиологии за этот период составил 1,5 на 100 тыс. новорожденных.
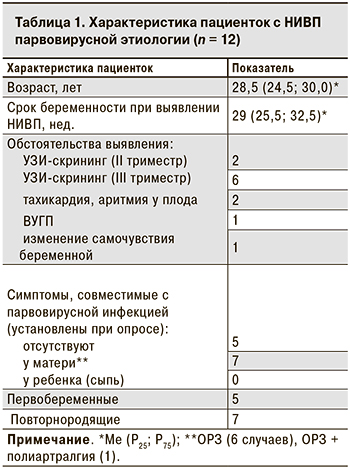
Беременные с парвовирусной водянкой плода были в возрасте от 20 до 39 лет (табл. 1). Ни у одной из них не было указаний на эпизоды острой экзантемной инфекции или контакт с пациентом с острой экзантемой в период беременности.
7 женщин перенесли ОРИ с субфебрильной лихорадкой (6 случаев) или без лихорадки (1 случай) за 2–14 нед. до выявления патологии плода (табл. 1). У 1 из них ОРИ сопровождалась артралгиями, что характерно для парвовирусной инфекции, однако лабораторного обследования для подтверждения диагноза в тот момент проведено не было. В 8 из 12 случаев водянка плода была обнаружена при проведении планового УЗИ-скрининга и только в 3 – при дополнительном УЗИ в связи с ухудшением самочувствия беременной (в 25 нед. гестации) или плода (в 30–32 нед.).
Наиболее часто при УЗИ наблюдались такие признаки водянки плода, как подкожный отек (83,3%), асцит (75,0%), плевральный и перикардиальный выпот (по 58,3%) (табл. 2).
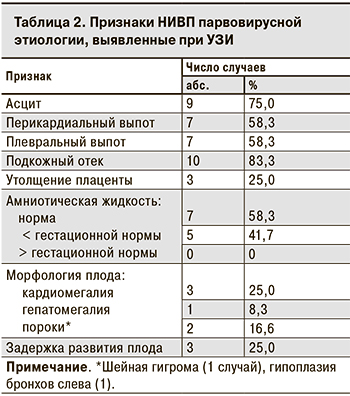 В случае ВУГП множественные признаки водянки были выявлены при патологоанатомическом исследовании, в то время как в заключении УЗИ была указана только ВУГП [10]. У 2 плодов при УЗИ наряду с подкожным отеком отмечался только гидроторакс, однако после рождения ребенка было выявлено скопление жидкости и в других серозных полостях, что свидетельствует о наличии определенных сложностей при проведении пренатальной УЗИ-диагностики.
В случае ВУГП множественные признаки водянки были выявлены при патологоанатомическом исследовании, в то время как в заключении УЗИ была указана только ВУГП [10]. У 2 плодов при УЗИ наряду с подкожным отеком отмечался только гидроторакс, однако после рождения ребенка было выявлено скопление жидкости и в других серозных полостях, что свидетельствует о наличии определенных сложностей при проведении пренатальной УЗИ-диагностики.
В 5 случаях беременность закончилась ВУГП в сроки от 19 до 28 нед., в остальных 7 – рождением недоношенный детей в 30–36 нед. Ме гестационного возраста при ВУГП была значительно меньше, чем в случаях, закончившихся рождением ребенка (24 нед. против 32; p = 0,01). Двое новорожденных погибли в неонатальном периоде. Таким образом, в 7 случаях водянка плода парвовирусной этиологии привела к неблагоприятному исходу беременности в 7 случаях, 5 детей были выписаны домой.
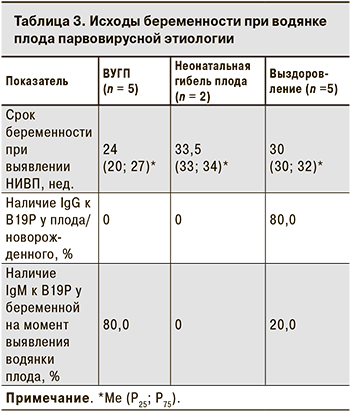 Только у 1 из 5 выживших детей водянка плода была выявлена в 26 нед. гестации. Было проведено внутриутробное переливание крови, которое привело к купированию симптомов водянки, однако в результате самопроизвольной родовой деятельности в 35 нед. родился недоношенный ребенок. В остальных 4 случаях признаки водянки отсутствовали при скрининговом УЗИ во II триместре, что свидетельствует о более позднем ее формировании.
Только у 1 из 5 выживших детей водянка плода была выявлена в 26 нед. гестации. Было проведено внутриутробное переливание крови, которое привело к купированию симптомов водянки, однако в результате самопроизвольной родовой деятельности в 35 нед. родился недоношенный ребенок. В остальных 4 случаях признаки водянки отсутствовали при скрининговом УЗИ во II триместре, что свидетельствует о более позднем ее формировании.
При неблагоприятном исходе беременности серологическое обследование было проведено лишь у 2 плодов, при этом специфические IgG отсутствовали, однако небольшое число обследованных не позволяет сделать заключение о распространенности противовирусных антител в таких случаях. Все 5 выживших детей были обследованы серологически, и 4 из них имели в сыворотке крови специфические IgG к В19Р (табл 3.). У 80,0% женщин с ВУГП обнаруживали специфические IgM, свидетельствующие о недавно перенесенной инфекции, Ме срока беременности в этих случаях составила 24 нед., что может свидетельствовать о более быстром формировании и тяжелом течении водянки плода при инфицировании во II триместре.
Обсуждение
В ходе исследования было установлено, что в 12 (23,5%) случаях этиологическим агентом заболевания являлся В19Р. Хотя в большинстве публикаций парвовирусная инфекция рассматривается как причина 10–16% случаев НИВП [11], согласно результатам 15-летнего наблюдения, проведенного во Франции, она обусловила 22% случаев [12], что практически совпадает с данными, полученными в Республике Беларусь.
Основным инструментом для подтверждения парвовирусной инфекции при НИВП служили молекулярные методы. Обнаружение вирусной ДНК было вдвое эффективнее в сравнении с выявлением IgM при обследовании беременных (12 против 5) и явилось единственным инструментом, позволившим верифицировать инфекцию у новорожденного или плода. К несомненным преимуществам молекулярных методов относится также возможность исследовать любой доступный биологический материал новорожденного или плода, включая патологоанатомические образцы [10]. Количественное определение содержания ДНК В19Р в биологических образцах в нашем исследовании не проводилось. Представленные в литературе данные свидетельствуют об отсутствии связи между количеством вируса и наличием симптомов у матери или плода. Кроме того, в большинстве случаев интерпретацию этих результатов затрудняет отсутствие точных данных о сроках заболевания на момент обследования [13].
IgM-антитела к В19Р не были обнаружены ни у одного из новорожденных, независимо от срока гестации на момент обследования, что подтверждает данные литературы о редком выявлении специфических IgM при внутриутробном инфицировании В19Р [13–15]. При этом в исследовании J. Weiffenbach с соавт. [16] у 3 новорожденных с парвовирусной водянкой плода IgM-антитела отсутствовали, несмотря на то что они выявлялись во время внутриутробного обследования в 25 нед. гестации [16]. Нельзя исключить, что подобная ситуация могла иметь место в ряде случаев и в нашем исследовании.
Особенность иммунного ответа на пренатальную парвовирусную инфекцию выражалась и в редком обнаружении у новорожденных/плодов специфических IgG, хотя этот тип антител не только формируется самим плодом, но и передается трансплацентарно от матери. Как и в других наблюдениях, мы не выявили корреляции между наличием противовирусных IgG у матери и новорожденного: из 7 серологически обследованных пар мать–ребенок положительный уровень IgG-антител имели все матери и лишь 4 (57,1%) новорожденных. Считается, что в наибольшей степени дефицит IgG при внутриутробной парвовирусной инфекции обусловлен повреждением плаценты и нарушением транспорта материнских антител к плоду [16]. В ряде публикаций [15, 17, 18] отмечено увеличение доли IgG-позитивных плодов с увеличением срока гестации. В нашем исследовании гестационный возраст новорожденных/плодов, имевших и не имевших специфических IgG, не различался и составлял 29–33 и 27–34 нед. соответственно. Однако то, что 4 из 5 выживших детей имели эти антитела, свидетельствует об их значимости для благополучного исхода.
Гестационный возраст плода на момент выявления парвовирусной водянки был в нашем исследовании достаточно большим, в 75,0% случаев он составлял от 25 до 33 нед., в то время как, по данным литературы, эта патология преимущественно выявляется ранее 25 нед. беременности [8]. Такое различие в определённой степени может быть обусловлено тем, что случаи ВУГП до 22 нед. в Республике Беларусь не подлежат обязательному патологоанатомическому исследованию, и какая-то часть случаев парвовирусной этиологии может оставаться нераспознанной1. Нельзя исключить, что это обстоятельство повлияло и на более низкую частоту выявления парвовирусной НИВП в Республике (1,5 на 100 тыс. новорожденных) в сравнении с установленной в многолетних исследованиях в США и Франции (2 и 3 на 100 тыс. новорожденных соответственно) [12, 19].
Полученные нами данные подтверждают наблюдения об отсутствии связи между наличием симптомов парвовирусной инфекции у матери и заболеванием плода [4, 12, 20–22]. Ни у одной из пациенток или контактных с ними лиц не было эпизодов острой экзантемы, и лишь половина женщин перенесли ОРИ в период беременности. Мы не наблюдали убедительной связи между развитием парвовирусной инфекции в период беременности и наличием в семье детей, что нередко расценивается как фактор риска инфицирования женщины, поскольку из 12 женщин у 5 текущая беременность была первой [12, 23–26]. Субклиническое течение и преобладание неспецифических симптомов обусловливают сложность своевременной диагностики парвовирусной инфекции во время беременности и в большинстве случаев не позволяют точно определить период между инфицированием и развитием водянки плода.
Тем не менее у 2 женщин в нашем исследовании можно с высокой долей вероятности говорить о сроках инфицирования. Одна из них на 16 нед. беременности перенесла ОРИ, сопровождавшуюся артралгиями, что характерно для парвовирусной инфекции у взрослых [27]. Водянка плода была выявлена на 30-й нед. беременности, то есть через 14 нед. после заболевания женщины. Хотя такой продолжительный инкубационный период, согласно данным литературы, наблюдается редко, он подтверждает важность длительного динамического наблюдения за женщиной, если парвовирусная инфекция была диагностирована во время беременности [11, 20, 28].
Во втором случае в 32 нед. беременности, при обнаружении у плода минимальных УЗИ-признаков водянки (изолированный гидроторакс), у матери была выявлена сероконверсия IgG-антител к В19Р. Поскольку специфические IgG формируются через 2–3 нед. после контакта с возбудителем, можно предполагать, что инфицирование произошло не ранее 28-й нед. Считается, что перенесенная в III триместре беременности парвовирусная инфекция является причиной 14–15% случаев ВУГП, однако крайне редко сопровождается формированием водянки. Тем не менее вероятность такого исхода подтверждает как наше наблюдение, так и описанные в литературе случаи развития водянки плода в 30–33 нед. гестации после инфицирования женщин в 24–27 нед. [9, 16, 29, 30].
Парвовирусная водянка плода преимущественно имеет неблагоприятный исход [21, 31], как это наблюдалось и в нашем исследовании, когда 7 (58,3%) из 12 беременностей закончились гибелью плода или новорожденного. ВУГП соответствовала более раннему сроку беременности при выявлении водянки (Ме – 24 нед.) в сравнении со случаями, когда беременность завершилась рождением недоношенного ребенка (Ме – 32 нед.) (р = 0,01). В нашем исследовании все женщины находились в одинаковых условиях ведения беременности, никаких изменений в тактику скриниговых обследований за 7 лет наблюдения внесено не было. С учетом этого с большой долей вероятности можно полагать, что более ранее выявление водянки плода соответствовало более раннему ее формированию. Это свидетельствует о более тяжелом течение водянки при ее формировании во II триместре беременности.
Мы изучали значимость только парвовирусной инфекции в этиологии НИВП в Республике Беларусь. Хотя, согласно данным литературы, В19Р принадлежит основная роль в структуре инфекционных агентов, вызывающих данное заболевание, несомненно, что расширение спектра диагностируемых возбудителей позволит получить более точные данные о вкладе инфекций в развитие НИВП [32, 33].
Выводы
- В течение 7 лет (2012–2018) в Республике Беларусь парвовирусная инфекция явилась причиной 24,0% исследованных случаев НИВП (12 заболевших). Средний многолетний показатель заболеваемости водянкой плода парвовирусной этиологии составил 1,5 на 100 тыс. новорожденных. Основным методом верификации диагноза явилось выявление ДНК В19Р в сыворотке крови беременных и разных видах биологического материала новорожденного или плода. Исследование IgM-антител играло вспомогательную роль при верификации диагноза у матери и было неинформативно для подтверждения инфицирования плода.
- Типичная клиническая картина парвовирусной инфекции крайне редко наблюдалась у беременных с водянкой плода, в связи с чем лабораторное обследование для верификации диагноза было проведено только после выявления патологии плода. У 8 из 12 пациенток выявление водянки явилось случайной находкой при проведении скринингового УЗИ, у остальных 4 – дополнительного УЗИ в связи с ухудшением самочувствия матери или плода. Длительность периода от инфицирования матери до выявления водянки плода составляла от 3 до 14 нед.
- Наиболее часто при УЗИ плода наблюдались такие признаки водянки, как подкожный отек (83,3%), асцит (66,7%), плевральный и перикардиальный выпот (по 58,3%). Неблагоприятно закончились 7 из 12 беременностей: в 5 случаях произошла ВУГП, в 2 – гибель новорожденного в неонатальном периоде. ВУГП соответствовала более раннему сроку гестации при выявлении водянки (Ме – 24 нед.) в сравнении со случаями, когда беременность завершилась рождением недоношенного ребенка (Ме – 32 нед.) (р = 0,01). Наличие специфических противовирусных IgG только у выживших новорожденных подтверждает важную роль иммунного ответа для благоприятного исхода при внутриутробном инфицировании В19Р.


