Грипп до настоящего времени остается актуальной инфекцией, способной преподносить неожиданные сюрпризы, несмотря на достижения в области вакцинопрофилактики [1–9] и большой выбор противогриппозных препаратов [10–13]. Доля острых респираторных вирусных инфекций (ОРВИ), в том числе гриппа, в структуре инфекционных болезней составляет до 92% [14–17].
Пандемия гриппа в эпидемический сезон 2009/2010 гг. показала, что риск развития осложнений, прежде всего внебольничной пневмонии, возникает в первую очередь у детей младшего возраста; беременных; пациентов любого возраста с хронической болезнью легких, заболеваниями сердечно-сосудистой системы, нарушениями обмена веществ, хроническими заболеваниями почек, печени, с определенными неврологическими состояниями, гемоглобинопатиями или иммунодефицитами; лиц в возрасте 60 лет и старше; пациентов с морбидным ожирением [18–23].
Следует отметить, что люди старше 60 лет – наиболее интенсивно увеличивающаяся часть населения в России, и эпидемическая значимость ее будет возрастать. К основным факторам риска, приводящим к летальным исходам у пожилых людей при заболевании гриппом, относят наличие сопутствующих заболеваний сердечно-сосудистый системы, болезни легких, нарушений обмена веществ, иммунодефицитов [4, 19, 24–26].
Проведение исследований, посвященных изучению эффективности противогриппозных вакцин у лиц старше 60 лет, является актуальным, так как позволяет обосновать необходимость и целесообразность вакцинопрофилактики гриппа у данной возрастной группы в преддверии эпидсезона.
Целью проведенного исследования было изучение безопасности, реактогенности, иммунологической и эпидемиологической эффективности применения отечественной адьювантной гриппозной тривалентной инактивированной полимер-субъединичной вакцины «Гриппол® плюс» у лиц 60 лет и старше.
Задачи исследования: изучить эффективность, безопасность вакцины «Гриппол® плюс» и оценить эпидемиологическую эффективность вакцинации у привитых в сравнении с группой непривитых.
Материалы и методы
Исследование проводили в эпидсезон 2012/2013 гг. на базе 4 клинических центров: МБУ «Центральная городская больница № 7» (Екатеринбург), ООО «АСКО-МЕД-ПЛЮС» (Барнаул), ГБОУ ВПО «Первый Санкт-Петербургский государственный медицинский университет имени академика И.П. Павлова» Минздрава России и ФГБУ «Государственный научно-исследовательский центр профилактической медицины» Минздрава России (Москва).
Дизайн исследования: открытое проспективное типа «случай–контроль», рандомизированное с ослеплением по конечным точкам. На первом этапе в исследование включали лиц обоего пола в возрасте 60 лет и старше, отказавшихся от вакцинации против гриппа. На втором этапе каждому из включенных участников подбирали по одному участнику, соответствующему по возрасту, полу и наличию хронических заболеваний в анамнезе и желающему пройти вакцинацию против гриппа.
 Все участвующие в исследовании (п = 480) были распределены на 2 группы: в 1-ю (п = 241) вошли лица, не привитые против гриппа, во 2-ю (п = 239) – привитые.
Все участвующие в исследовании (п = 480) были распределены на 2 группы: в 1-ю (п = 241) вошли лица, не привитые против гриппа, во 2-ю (п = 239) – привитые.
В исследование включали лиц, удовлетворяющих следующим критериям: подписавшие информированное согласие участника; входящие в возрастную группу 60 лет и старше; не вакцинированные в последние 6 мес., предшествовавших вакцинации; не имеющие противопоказаний, предусмотренных инструкцией по применению вакцины «Гриппол® плюс».
Критериями исключения являлись: участие в другом клиническом исследовании; наличие острых инфекционных и соматических заболеваний или обострение хронических заболеваний в период менее 1 мес. с момента клинического выздоровления или ремиссии; наличие декомпенсированных заболеваний, которые могли повлиять на проведение исследования (органические поражения центральной нервной системы, декомпенсированная патология сердечно-сосудистой системы, острая почечная или печеночная недостаточность, онкологические заболевания, ВИЧ и ВИЧ-ассоциированные заболевания); прием иммуносупрессивных препаратов; лактозная недостаточность. Противопоказанием к вакцинации являлись аллергическая реакция на белок куриного яйца и тяжелые аллергические реакции на предыдущие введения вакцины от гриппа в анамнезе.
Отказаться от участия в исследовании можно было по желанию на любом из этапов.
Отечественная гриппозная тривалентная инактивированная полимер-субъединичная вакцина «Гриппол® плюс» представляет собой протективные антигены (гемагглютинин и нейраминидаза) вирусов гриппа типа А и В, выращенных в аллантоисе куриных эмбрионов, связанные с водорастворимым высокомолекулярным иммуноадъювантом Полиоксидоний®. Одна иммунизирующая доза (0,5 мл) содержит по 5 мкг гемагглютинина эпидемически актуальных штаммов вируса гриппа подтипов А (H1N1 и H3N2) и типа В, 500 мкг иммуноадъюванта Полиоксидоний® в фосфатно-солевом буфере. Вакцина не содержит консерванта.
У привитых лиц перед вакцинацией проводили забор крови. Вакцину вводили внутримышечно в дельтовидную мышцу. Для выявления и регистрации немедленных реакций на введение вакцины все привитые находились под медицинским наблюдением в течение 30 мин. В последующие 5 дней за привитыми пациентами велось медицинское наблюдение для выявления местных и общих реакций. Через 21–28 дней, а затем через 6 мес. после вакцинации проводили забор крови для оценки иммунологической эффективности. В течение 6 мес. осуществляли активное наблюдение за привитыми для выявления симптомов ОРВИ. Информация о посещениях врача и результатах исследований отражалась в индивидуальной регистрационной и амбулаторной картах.
В течение всего срока наблюдения за пациентами обеих групп собирали данные о соматической и инфекционной заболеваемости.
Клиническую эффективность вакцинации оценивали по разнице частоты госпитализаций по неотложным показаниям при заболеваниях сердечно-сосудистой системы, рецидивирующих заболеваниях респираторного тракта (ларинготрахеиты, бронхиты, пневмонии и др.) в группах наблюдения.
Частоту ОРВИ и обострений хронических респираторных заболеваний учитывали по первичной медицинской документации (амбулаторная карта в поликлинике, где отражена информация о посещениях больными врача в поликлинике).
Уровень безопасности и реактогенности вакцины для лиц старше 60 лет оценивали по наличию/отсутствию общих (повышение температуры тела, недомогание, головная и мышечная боль, артралгия, насморк, нарушение сна, аппетита, тошнота, рвота, першение в горле) и местных (боль и зуд в месте инъекции, покраснение, припухлость) реакций, а также нежелательных явлений.
Для оценки антигенной активности иммунобиологического препарата исследовали парные сыворотки в РТГА (реакция торможения гемагглютинации) по общепринятой методике (до вакцинации, на 21–28-й день и через 6 мес. после вакцинации), определяя уровень антигемагглютинирующих антител. Результаты сравнивали с требованиями международных стандартов CPMP EMEA, CPMP/EWP/1045/01 (для лиц старше 60 лет гриппозная вакцина считается иммуногенной, если соответствует, по крайней мере, одному из критериев: уровень серопротекции ≥ 60%, уровень сероконверсий ≥ 30%, кратность нарастания титра антител ≥ 2,0).
Статистические методы обработки полученных результатов: количественные признаки оценивали с помощью среднего арифметического значения ± стандартное отклонение (M ± m). При анализе титров антител описание количественных показателей проводили с помощью средних геометрических значений. Качественные признаки представлены в виде частоты событий (сероконверсии, серопротекции, частоты встречаемости ОРЗ/гриппа и других заболеваний и т. п.) в %. Сравнение частоты событий в несвязанных выборках выполнено с помощью критерия χ2 с поправкой по Йейтсу при множественных сравнениях. Статистически значимыми считались различия при p < 0,05.
 Результаты
Результаты
Через 30 мин. после вакцинации ни у одного вакцинированного не было отмечено ни местных, ни общих реакций.
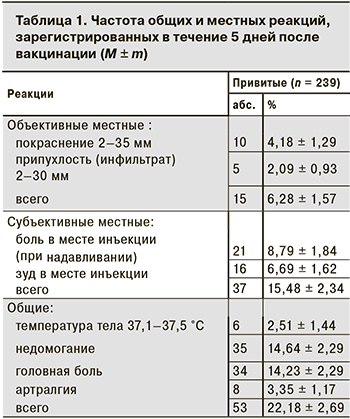
Объективные местные реакции регистрировали у 6,28 ± 1,57% привитых. Все они были слабо выражены и являлись нормальными вакцинальными реакциями (табл. 1). Не было зафиксировано и осложненных местных реакций.
Поствакцинальные реакции не вызывали клинически значимого нарушения самочувствия и не требовали медицинского вмешательства. Не было ни одного случая сильных общих вакцинальных реакций или поствакцинальных осложнений.
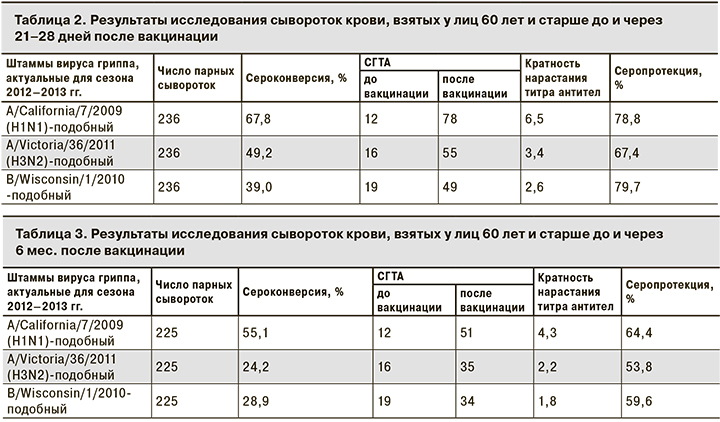
Оценка результатов исследования иммунологической эффективности вакцины «Гриппол® плюс» в парных сыворотках до вакцинации и через 21–28 дней после нее показала соответствие международным критериям СРМР, предъявляемым к вакцинным препаратам для возрастной группы 60 лет и старше, в отношении всех 3 штаммов вируса гриппа (табл. 2).
К штамму вируса гриппа A/California/7/2009(H1N1) у 78,8% участников в крови определялся защитный уровень антител. Показатель сероконверсии составил 67,8% при СГТА 1/78, кратность нарастания титра антител по сравнению с довакцинальным уровнем составила 6,5 раза.
К штамму вируса гриппа A/Victoria/36/2011(H3N2) защитный титр антител определяли в 67,4% случаев, показатель сероконверсии составил 49,2% при СГТА 1/55, что в 3,4 раза выше довакцинального уровня.
К штамму вируса гриппа В/Wisconsin/1/2010 защитный уровень антител определяли у 79,7% привитых, показатель сероконверсии составлял 39,0% при СГТА 1/49, по сравнению с довакцинальным уровнем СГТА увеличилась в 2,6 раза.
Через 6 мес. после вакцинации все показатели иммуногенности снизились по сравнению с показателями на 21–28-й день, что является закономерным не только для лиц пожилого возраста. Согласно критериям СРМР, через 6 мес. после вакцинации вакцина «Гриппол® плюс» соответствовала международным стандартам: к штамму A/ California/7/2009(H1N1) – по 3 показателям, к штаммам A/Victoria/36/2011(H3N2) и В/Wisconsin/1/2010 – по 1 (табл. 3).
К штамму вируса гриппа A/California/7/2009(H1N1) защитный уровень антител сохранялся у 64,4% участников, показатель сероконверсии составлял 55,1% при СГТА 1/51, что выше довакцинального уровня в 4,3 раза.
К штамму вируса гриппа A/Victoria/36/2011(H3N2) показатели серопротекции и сероконверсии составили 53,8 и 24,2%, соответственно при СГТА 1/35, что в 2,2 раза выше, чем до вакцинации.
К штамму В/Wisconsin/1/2010 показатели серопротекции и сероконверсии составили 59,6 и 28,9% соответственно при СГТА 1/34, что в 1,8 раза выше, чем до вакцинации.
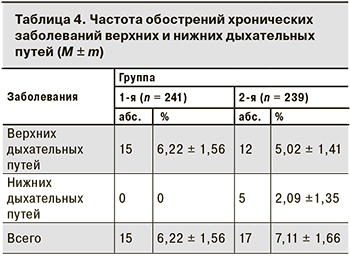
В течение 6 мес. от начала исследования у участников выявляли и регистрировали ОРВИ и обострение хронических заболеваний верхних дыхательных путей.
Случаи заболевания ОРВИ за 6 мес. поствакцинального периода в 1-й группе регистрировали в 2,08 раза чаще, чем во 2-й (17,43 ± 4,88 и 8,37 ± 3,58% соответственно; р < 0,05). По частоте обострений хронических заболеваний респираторного тракта показатели 1-й и 2-й групп были сопоставимы (р > 0,05; табл. 4).
Обсуждение
Результаты проведенного исследования убедительно продемонстрировали иммунологическую эффективность применения у лиц старше 60 лет отечественной субъединичной адюъвантной вакцины со сниженным содержанием антигена (5 мкг) в сравнении с препаратами, содержащими по 15 мкг каждого антигена, представленными в других исследованиях [2, 5, 16].
Показатели иммунологической эффективности (серопротекция, сероконверсия, кратность нарастания титра антител) через 21–28 дней после вакцинации соответствовали критериям CPMP и, несмотря на снижение уровня антител к вирусам гриппа, сохранялись на протективном уровне в течение 6 мес. после вакцинации.
Эпидемиологическую эффективность вакцинации против гриппа оценивали по разнице заболеваемости всем комплексом ОРВИ среди привитых и непривитых в течение 6 мес. Участники в 1-й группе болели ОРВИ достоверно чаще.
Кроме того, мы проанализировали частоту обострений хронических заболеваний верхних и нижних дыхательных путей в обеих группах. Разницы в показателях между 1-й и 2-й группами не установлено.
Выводы
- Вакцина «Гриппол® плюс» продемонстрировала высокий профиль ареактогенности и иммунологической эффективности при иммунизации лиц старше 60 лет. Активно выявленные реакции (местные и общие) расценивались как слабые и не требовали медицинского вмешательства. Через 21–28 дней и 6 мес. после вакцинации показатели сероконверсии, серопротекции и кратность нарастания титров антител к штаммам гриппа A/California/7/2009(H1N1), A/ Victoria/36/2011(H3N2) и В/Wisconsin/1/2010 соответствовали международным критериям СРМР.
- Показана эпидемиологическая эффективность вакцины при иммунизации лиц старше 60 лет. В течение 6 мес. наблюдения после вакцинации заболеваемость ОРВИ у привитых была в 2,08 раза ниже, чем у непривитых.


