Широкое использование антиретровирусной терапии (АРТ) привело к значительному снижению смертности среди больных ВИЧ-инфекцией. Однако побочные эффекты АРТ ограничивают выбор схемы у определенных категорий пациентов, ведут к несоблюдению режима приема и необходимости смены схемы терапии у 25% больных уже в первый год лечения, что существенно снижает эффективность терапии, повышает ее стоимость в будущем [1].
Около 70% ВИЧ-инфицированных пациентов в РФ начинают АРТ с наиболее дешевых схем, обладающих, несмотря на высокую эффективность, широким спектром побочных эффектов. Например, зидовудин/ламивудин (ZDV/3TC – комбивир®), который в РФ получают около 46% пациентов, вызывает анемию, нейтропению, повышение уровня печеночных ферментов и лактатацидоз; эфавиренз (ЕFV – стокрин®; 28,3% пациентов) – головокружение, головную боль, тяжелую депрессию, агрессивное поведение; лопинавир (LPV/r – калетра®; 35,8% пациентов) – диарею, тошноту, рвоту, дислипидемию, повышает риск развития кардиоваскулярных расстройств [2]. Примерно 30% больных, получающих АРТ, нуждаются в смене терапии из-за ее непереносимости: из-за выраженных побочных эффектов пациенты перестают лечиться, поэтому средняя длительность приема схем первой линии АРТ у больных ВИЧ-инфекцией в России не превышает 2–3 лет, что в 2–2,5 раза меньше, чем в странах ЕС. Другим недостатком таких схем является неудобство их ежедневного пожизненного приема: в среднем российский пациент получает АРТ, состоящую из 5–6 таблеток и требующую приема 2–3 раза в сутки [2].
 Это обусловливает низкую приверженность пациентов к лечению и приводит к развитию резистентности и вирусологической неудаче текущей схемы АРТ. Неудача терапии требует смены схемы АРТ на более дорогостоящую, тем самым значимо удорожает общую стоимость ведения больного ВИЧ-инфекцией, а также может привести к отсутствию выбора резервных схем терапии в будущем.
Это обусловливает низкую приверженность пациентов к лечению и приводит к развитию резистентности и вирусологической неудаче текущей схемы АРТ. Неудача терапии требует смены схемы АРТ на более дорогостоящую, тем самым значимо удорожает общую стоимость ведения больного ВИЧ-инфекцией, а также может привести к отсутствию выбора резервных схем терапии в будущем.
У ВИЧ-инфицированных лиц основное заболевание часто сопровождается сопутствующей патологией. Например, сердечно-сосудистые заболевания встречаются у 7,5% больных [3], нарушения липидного обмена – у 70–80% [4], коинфекции ВИЧ и вирусами гепатитов B (ВГВ) и С (ВСГ) – у 60–70% [5].
У пациентов с сопутствующей патологией чаще развиваются ранние побочные эффекты АРТ, которые в России выявляются в среднем у 42% больных ВИЧ-инфекцией и проявляются уже в первые 6 мес. терапии, а примерно у 10% пациентов они требуют изменения схемы лечения или его отмены. Например, частота побочных эффектов со стороны ЦНС при приеме ЕFV, одного из часто используемых препаратов, через 6 мес. составляет около 22%. При использовании LPV частота нежелательных явлений (НЯ) со стороны ЖКТ достигает 52,2% [6].
Согласно рекомендациям ВОЗ [7], больным ВИЧ-инфекцией при выборе схемы АРТ первой линии рекомендовано применение наименее токсичных препаратов с сохранением принципа простоты приема, то есть с включением их в состав комбинированных лекарственных средств с фиксированными комбинациями доз. Комбинированные режимы, в том числе и полные схемы АРТ в 1 таблетке (single tablet regimen – STR), рекомендованы в качестве старта терапии у больных ВИЧ-инфекцией основными международными руководствами EACS, BHIVA, DHHS [8–10].
Один из представителей класса STR – рилпивирин/тенофовир/эмтрицитабин (RPV/TDF/FTC – эвиплера) был зарегистрирована в РФ в декабре 2013 г. (РУ №ЛП-002324) и на настоящий момент является единственным в России препаратом, содержащим полный режим АРТ в 1 таблетке с приемом всего 1 раз в сутки. RPV/TDF/FTC внесен и в российские клинические рекомендации по ведению больных ВИЧ-инфекцией [11, 12].
В сентябре 2015 г. препарат был включен в перечень жизненно необходимых и важнейших лекарственных препаратов для медицинского применения на 2016 г.
RPV/TDF/FTC показан для лечения инфекции, вызванной ВИЧ-1, в качестве АРТ первой линии у взрослых пациентов, имеющих показатели РНК ВИЧ-1 ≤ 100 000 копий/мл. Препарат может применяться у пациентов с вирусной нагрузкой (ВН) ≤ 100 000 копий/мл, ранее не получавших АРТ, а также с целью переключения при непереносимости предыдущих схем терапии или с целью упрощения терапии.
В ходе международных клинических исследований было продемонстрировано, что вирусологическая эффективность RPV/TDF/FTC сопоставима с «золотым стандартом» лечения ВИЧ-инфекции – ЕFV: у 80 и 73% пациентов соответственно наблюдалась вирусологическая супрессия через 96 недель АРТ [13, 14].
Было установлено, что RPV/TDF/FTC обладает благоприятным профилем безопасности и переносимости: частота НЯ со стороны ЦНС на 20% ниже, чем при приеме ЕFV [13, 14]. Также показано минимальное влияние комбинированного препарата на липидный профиль при назначении его в качестве старта АРТ и снижение уровней общего холестерина [на 0,65 ммоль/л по сравнению с 0,03 ммоль/л при приеме ингибиторов протеазы (ИП)], триглицеридов (снижение на 0,6 ммоль/л по сравнению с повышением на 0,03 ммоль/л при продолжении приема ИП) при переключении с ИП [15, 16].
Хорошая переносимость и удобство приема препарата обеспечивают повышение качества жизни пациента, что является одним из определяющих факторов при выборе схемы АРТ. Высокий уровень безопасности RPV/TDF/FTC позволяет назначать препарат пациентам, которым противопоказаны другие схемы АРТ: лицам с заболеваниями ЦНС, сердечно-сосудистыми заболеваниями, коинфекцией ВИЧ и ВГВ/ВГС [17].
Необходимо отметить, что за счет входящих в состав комбинированного препарата TDF и FTC он эффективен в отношении как ВИЧ-инфекции, так и гепатита B, что делает возможным применение его у пациентов с коинфекцией ВИЧ/ВГВ.
В ходе проведенного в России фармакоэкономического исследования [18] было показано, что внедрение RPV/TDF/FTC в практику российского здравоохранения в 5-летней перспективе приведет к снижению количества новых случаев ВИЧ-инфекции на 13%, смертности среди больных ВИЧ-инфекцией – на 18%, повышению продолжительности их жизни – на 14%, снижению госпитализаций – на 25% по сравнению с наиболее часто назначаемыми режимами АРТ, включающими 3 препарата (на основе LPV, ЕFV и базовой терапии).
Цель нашего исследования – оценка эффективности, безопасности и переносимости комбинированного препарата RPV/TDF/FTC у российских пациентов с вирусной нагрузкой (ВН) < 100 000 копий/мл.
Материалы и методы
Исследование был многоцентровым, нерандомизированным, открытым, проводилось в условиях реальной клинической практики, поэтому в нем приняли участие как пациенты, которые получали терапию впервые, так и те, кому требовалось изменение схемы лечения из-за развития НЯ или с целью упрощения терапии.
В исследование были включены 29 взрослых больных ВИЧ-инфекцией, которые в течение 6 мес. получали RPV/TDF/FTC – комбинированный препарат с фиксированной комбинацией доз.
Все пациенты состояли на диспансерном учете: 7 – в Центре по профилактике и борьбе со СПИД и инфекционными заболеваниями (Санкт-Петербург), 6 – в Ямало-Ненецком окружном центре профилактики и борьбы со СПИД (г. Ноябрьск), 5 – в Волгоградском областном центре по профилактике и борьбе со СПИД и инфекционными заболеваниями, 3 – в Центре по профилактике и борьбе со СПИД и инфекционными заболеваниями Ленинградской области (Санкт-Петербург), 3 – в Нижегородском областном центре по профилактике и борьбе со СПИД и инфекционными заболеваниями (Нижний Новгород), 3 – в Клиническом центре профилактики и борьбы со СПИД Минздрава Краснодарского края (Краснодар), 2 – в Республиканской клинической инфекционной больнице Минздрава России (Санкт-Петербург, пос. Усть-Ижора).
Основными критериями включения в исследование были наличие подтвержденного диагноза ВИЧ-инфекции, возраст старше 18 лет, уровень РНК ВИЧ ≤ 100 000 копий/мл на момент включения.
Основными критериями исключения из исследования были беременность и кормление грудью, наличие у пациента состояния (включая злоупотребление алкоголем, лекарственную зависимость и др.), препятствующего соблюдению схемы терапии, выраженная сопутствующая патология (сердечно-сосудистая, эндокринной системы, почек и т. д.).
Материалом для исследования послужили результаты обследования, медицинские карты амбулаторных больных, выписки с предшествующих этапов лечения.
Среди пациентов преобладали мужчины (58,7%). Возраст больных колебался от 23 лет до 61 года (в среднем – 40 лет), при этом 62,1% пациентов были старше 35 лет.
Длительность ВИЧ-инфекции от постановки диагноза до начала АРТ в среднем составляла 3,45 года. Исходное количество CD4+-лимфоцитов – в среднем 527 клеток/мкл [интервал 51–2722 кл/мкл], из них только у 3 пациентов оно было < 200 клеток/мкл. Перед началом АРT большинство пациентов имели низкую ВН (у 86,2% больных РНК ВИЧ была < 500 копий/ мл). Интервал составил 20–88 300 копий/ мл. Стадии ВИЧ-инфекции 2А, 2В (острая ВИЧ-инфекция), 3 (субклиническая) и 4А имели 82,8% пациентов, стадию 4Б и 4В – 17,2% (табл.1).
До начала приема RPV/TDF/FTC в качестве третьего препарата 55,3% пациентов получали ИП, при этом наиболее распространенным был LPV/r, далее шли DRV/r и ATV/r. 27,7% пациентов получали ННИОТ, среди которых на первом месте был EFV (17,3%). Среди НИОТ больным ВИЧ-инфекцией чаще всего назначали комбинированные препараты ABC/3TC (38%), затем ZDV/3TC (34,5%) и TDF/FTC (13,8%).
Основными причинами перевода пациентов с описанных схем на прием RPV/TDF/FTC явились токсичность и необходимость упрощения терапии (см. табл.1).
Анализ клинически выраженных НЯ проводили у всех пациентов. Безопасность и переносимость схем лечения оценивали по частоте клинических НЯ, связанных с терапией, и изменению лабораторных показателей.
 Среди побочных эффектов, приведших к необходимости назначения RPV/TDF/FTC, наиболее часто встречались НЯ со стороны ЦНС (снижение настроения, головокружение, галлюцинации, дезориентация в пространстве, сновидения), вызванные приемом EFV. Дислипидемия и нарушения со стороны ЖКТ, как правило, регистрировалась на фоне приема ИП. Иктеричность склер, жалобы на желтушность кожных покровов обуславливали необходимость отмены АТV.
Среди побочных эффектов, приведших к необходимости назначения RPV/TDF/FTC, наиболее часто встречались НЯ со стороны ЦНС (снижение настроения, головокружение, галлюцинации, дезориентация в пространстве, сновидения), вызванные приемом EFV. Дислипидемия и нарушения со стороны ЖКТ, как правило, регистрировалась на фоне приема ИП. Иктеричность склер, жалобы на желтушность кожных покровов обуславливали необходимость отмены АТV.
У всех больных диагноз ВИЧ-инфекции был установлен на основании клинико-эпидемиологических данных и подтвержден положительным результатом исследования на антитела к ВИЧ методом иммуноблотинга. Стадию ВИЧ-инфекции определяли в соответствии с клинической классификацией ВИЧ-инфекции, утвержденной приказом Минздравсоцразвития РФ № 166 от 17.03.2006 г.
Клинические методы исследования включали сбор анамнеза и жалоб, осмотр и физикальное обследование. Лабораторные методы включали общеклинический и биохимический анализ крови по общепринятой методике; серологическое исследование сыворотки крови на маркеры гепатитов B и С, иммунологическое исследование субпопуляции CD4+-лимфоцитов; молекулярно-биологическое исследование уровня РНК ВИЧ-1 в плазме крови методом ПЦР, а также инструментальные методы (УЗИ органов брюшной полости, электрокардиографию, рентгенографию органов грудной клетки).
Для анализа метаболических нарушений определяли концентрации общего холестерина, ЛПВП, ЛПНП, триглицеридов.
Основным критерием оценки (первичная точка) эффективности явилась доля пациентов с неопределяемой ВН через 6 мес. приема RPV/TDF/FTC. Дополнительные критерии оценки (вторичные точки) эффективности: динамика ВН, количества CD4+-лимфоцитов через 6 мес. от начала лечения, частота и характер НЯ.
Статистическую обработку полученных данных осуществляли при помощи программ Microsoft Office Excel. Определяли среднее значение, стандартное отклонение, медиану. Сравнение средних показателей производили с помощью стандартных методов вариационной статистики. Для оценки значимости различий применяли t-критерия Стьюдента (пригодный для небольших выборок). Различия считались достоверными при p < 0,05.
Результаты и обсуждение
В анализ результатов по динамике ВН и уровня CD4+-лимфоцитов через 6 мес. от начала приема RPV/TDF/FTC были включены данные 22 пациентов, из которых только у одного больного наблюдалось повышение уровня РНК ВИЧ от исходного, у остальных пациентов ВН сохранялась на прежнем уровне или снизилась (6 больных).
Уменьшение ВН на фоне приема RPV/TDF/FTC сопровождалось ростом количества CD4+-лимфоцитов: средний прирост через 24 недели составил 50,5 клеток/мкл или 6% (см. рисунок).
У пациентов не отмечено каких-либо НЯ при приеме препарата в течение 24 недель (в том числе и со стороны ЦНС, ЖКТ). Только у 1 пациента уровень креатинина через 6 мес. достиг порогового значения 100 мкмоль/л. Еще у 1 больного наблюдалось повышение уровня АСТ, превышающее верхнюю границу нормы в 1,5 раза, и АЛТ – в 1,8 раза.
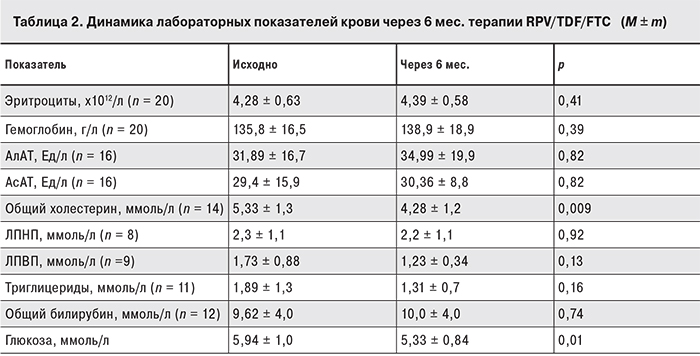
У всех пациентов сохранялись стабильными показатели гемограммы (эритроциты, гемоглобин), наблюдалась тенденция к снижению уровней общего холестерина, триглицеридов (p = 0,009), глюкозы. Не выявлено значимого повышения уровней печеночных трансаминаз и общего билирубина по сравнению с исходными значениями.
Результаты проведенного исследования показали вирусологическую и иммунологическую эффективность полного режима АРТ в 1 таблетке.
Полученные данные подтвердили результаты регистрационных исследований III фазы STAR и SPIRIT, свидетельствующие о высоком уровне эффективности RPV/TDF/FTC, сравнимом со схемами, содержащими EFV (STAR) и ИП (SPIRIT) [13, 15].
Положительная динамика прироста количества CD4+-лимфоцитов наблюдалась у большинства пациентов в течение 24 недель приема препарата.
Необходимо отметить, что у больных ВИЧ-инфекцией ранее, на фоне приема схем АРТ, включающих EFV, возникали побочные эффекты со стороны ЦНС, а на фоне приема ИП – нарушения липидного обмена и НЯ со стороны ЖКТ, то после перехода на RPV/TDF/FTC этих побочных эффектов не отмечено.
Несмотря на то, что у 41,4 % пациентов ВИЧ-инфекция сочеталась с ХГС, а у 6,9% – с ХГВ, за период наблюдения не было выявлено значимого повышения уровня печеночных ферментов и общего билирубина, что свидетельствует об отсутствии отрицательного влияния комбинированного препарата RPV/TDF/FTC на функциональное состояние печени.
Длительный прием АРТ, особенно у коинфицированных пациентов, получающих сопутствующую терапию, повышает риск развития фиброза печени. Сопутствующий хронический вирусный гепатит – независимый фактор риска развития гепатотоксичности антретровирусных препаратов, затрудняющий лечение пациентов с ВИЧ. В схемах терапии коинфицированных пациентов крайне необходим подбор препаратов с минимальной токсичностью, в том числе в отношении печени [6].
RPV/TDF/FTC благодаря своим преимуществам (1 таблетка 1 раз в сутки, высокая эффективность, благоприятный профиль безопасности и переносимости) является оптимальным выбором АРТ первой линии как для пациентов с ВН ≤ 100 000 копий/мл, ранее не получавших АРТ, так и для пациентов с непереносимостью предыдущих схем (например, на основе ННИОТ, ИП) или нуждающихся в упрощении режима терапии (например, переключение со схем на основе ралтегравира – RAL). Отсутствие отрицательного влияния на липидный профиль, значимых межлекарственных взаимодействий позволяют назначать RPV/TDF/FTC пациентам с сопутствующими заболеваниями сердечно-сосудистой системы, хроническими вирусными гепатитами [17].
Важно отметить, что больным с нарушениями липидного и углеводного обмена необходимо избегать назначения EFV и LPV в схемах АРТ для предотвращения развития сердечно-сосудистой патологии [2]. Таким пациентам необходимо подбирать схемы АРТ, компоненты которых оказывают наименьшее влияние на уровни липидов, с минимальным спектром НЯ. Как вариант им может быть показано назначение RPV/TDF/FTC.
Таким образом, первые данные применения RPV/TDF/FTC (эвиплера) у 29 российских пациентов в течение 6 мес. продемонстрировали высокую вирусологическую и иммунологическую эффективность препарата у пациентов с исходной ВН < 100 000 копий/мл, благоприятный профиль безопасности, отсутствие негативного влияния на функцию печени [в том числе хорошую переносимость у пациентов с коинфекцией ВИЧ/ВГВ(ВГС)] и липидный профиль.


