С внедрением в практику высокоактивной антиретровирусной терапии (АРВТ) перед практическим врачом ежедневно встает вопрос выбора оптимальной схемы лечения ВИЧ-инфицированных пациентов. Чем шире выбор предлагаемых антиретровирусных препаратов, тем выше возможность более оптимального и индивидуального подхода к назначению АРВТ у конкретного больного.
В России зарегистрировано 8 препаратов из группы ингибиторов протеазы (ИП), в их числе фосампренавир – FPV (телзир, компания ViiV Healthcare, Великобритания), который был одобрен в 2006 г. для лечения пациентов, как ранее не получавших, так и получавших АРВТ. Рекомендуемые режимы приема: FPV без ритонавира – 1400 мг 2 раза в сутки, либо FPV, бустированный ритонавиром (FPV/r) – 700/100 мг 2 раза в сутки; 1400/200 мг один раз в сутки и 1400/100 мг один раз в сутки. Однократный режим дозирования при применении как небустированного FPV, так и в комбинации с ритонавиром не рекомендуется у взрослых, ранее получавших терапию ИП [1].
Было проведено несколько крупных исследований, в которых доказана эффективность и безопасность FPV по сравнению с другими ИП [2–5].

В исследовании ALERT сравнивали эффективность и безопасность однократного (в сутки) приема FPV/r (1400/100 мг) и атазанавира/ритонавира (ATV/r; 300/100 мг) в комбинации с тенофовиром/эмтрицитабином (TDF/FTC) у 106 пациентов, ранее не получавших АРВТ. По результатам ITT-анализа, через 48 нед. терапии вирусная нагрузка (ВН) < 400 копий/мл была зарегистрирована у 79% из 53 пациентов, получающих FPV/r, и у 87% из 53 пациентов, получающих ATV/r (p = 0,3). Отмечена меньшая частота нежелательных явлений на фоне приема FPV/r из-за отсутствия повышения уровня сывороточного билирубина. Содержание липидов изменялось одинаково в обеих терапевтических группах [5].
В исследовании KLEAN было проведено сравнение по критерию «не меньшей эффективности» режимов приема FPV/r (700/100 мг) и лопинавира/ритонавира (LPV/r; 400/100 мг 2 раза в сутки) в комбинации с абакавиром/ламивудином (ABC/3ТС) у 877 "наивных" ВИЧ-инфицированных пациентов в течение 48 нед. Данное исследование было продлено до 144 нед. для 199 пациентов. На протяжении 144 нед. терапии обе схемы показали высокую и сравнимую эффективность, безопасность и хорошо переносились. Липидный профиль оставался относительно стабильным в обеих группах на протяжении 96 и 144 нед. терапии [2, 3].
В исследовании NEAT было продемонстрировано превосходство не усиленного ритонавиром FPV по сравнению с нелфинавиром (NFV) [4]. Последующее длительное наблюдение за подгруппой из 211 человек, продолживших прием FPV/r 1 раз в сутки, выявило, что у 139 (66%) пациентов спустя 120 нед. после завершения исследования ВН сохранялась на уровне < 50 копий/мл [6].
В последние годы внимание исследователей привлекает изучение безопасности FPV/r у пациентов с сопутствующей патологией печени [7, 8].
N. Merchante и соавт. [7] оценивали гепатотоксичность схем АРВТ, содержащих FPV/r, у 117 ВИЧ-инфицированных пациентов с коинфекцией вирусом гепатита С (ВГС). При этом основным оцениваемым показателем являлась частота повышения активности печеночных ферментов (ПАПФ) 3–4-й степени в течение 1 года от начала лечения. За время наблюдения ПАПФ 3–4-й степени возникли у 11 (9%) пациентов (95% ДИ 4,1–14,6). В исследовании принимали участие пациенты с исходным циррозом печени (n = 20; 21%), ни у одного из них не возникло ПАПФ 3–4-й степени. Кроме того, наличие выраженного фиброза и цирроза печени не влияло на частоту гепатотоксичности. По мнению авторов, схемы АРВТ с FPV/r 1 раз в сутки можно считать безопасными для пациентов с ВИЧ/ВГС, включая больных с циррозом печени [7].
J. Pineda и соавт. [8] оценивали частоту гепатотоксичности схем с FPV/r у 636 пациентов, их них у 341 (54%) были обнаружены антитела к ВГС, 38 (5,6%) человек являлись носителями HBsAg. У 93 (27%) пациентов, серопозитивных по анти-ВГС, соотношение уровня АсАТ и числа тромбоцитов (индекс APRI) было > 1,5, что соответствует значительному фиброзу печени. Медиана периода наблюдения составила 6,91[0,46–20,66] мес., в течение этого времени у 3 (0,47%) пациентов повысился уровень АлАТ ≥ 3-й степени. Частота повышения уровня АлАТ ≥ 3-й степени у пациентов, серопозитивных по анти-ВГС, составила 0,58%, а у носителей HBsAg – 2,63%. Не было выявлено ни одного пациента с индексом APRI ≥ 1,5 и повышением уровня АлАТ ≥ 3 степени. Таким образом, частота нежелательных явления со стороны печени у пациентов, получавших FPV/r в составе комбинированной АРВТ, оказалась низкой даже у пациентов с ВИЧ/ВГС, а также с выраженным фиброзом печени.
Международные рекомендации по лечению ВИЧ-инфекции [Европейского клинического общества по СПИДу (EACS), Международного общества по изучению СПИДа (IAS-USA), Департамента по здравоохранению и социальному развитию США (DHHS)] рассматривают FPV в комбинации с препаратами класса НИОТ в качестве альтернативного режима первой линии терапии [9–11].
 Согласно «Протоколам диспансерного лечения и наблюдения больных ВИЧ-инфекцией», разработанным Национальным научным обществом инфекционистов России под редакцией акад. РАМН В.В. Покровского [12], FPV рекомендуется в качестве 3-го компонента в комбинации с препаратами класса НИОТ в альтернативных режимах первого ряда и во второй линии АРВТ [12].
Согласно «Протоколам диспансерного лечения и наблюдения больных ВИЧ-инфекцией», разработанным Национальным научным обществом инфекционистов России под редакцией акад. РАМН В.В. Покровского [12], FPV рекомендуется в качестве 3-го компонента в комбинации с препаратами класса НИОТ в альтернативных режимах первого ряда и во второй линии АРВТ [12].
Основными преимуществами FPV являются удобство применения без связи с приемом пищи, благоприятный профиль лекарственных взаимодействий с препаратами для лечения патологии желудочно-кишечного тракта (ЖКТ) (антациды, антагонисты Н2-гистаминовых рецепторов, ингибиторы протонного насоса), а также безопасность для пациентов с сопутствующими заболеваниями печени и ЖКТ.
В связи с тем, что исследования схем с FPV выполнены зарубежными авторами, целесообразно обобщить опыт применения и оценить эффективность и влияние на печень данного ИП у российских пациентов.
Цель исследования – оценить эффективность и гепатотоксичность схем АРВТ с FPV/r в дозе 1400/100 мг 1 раз в сутки в течение 48 нед.
Материалы и методы
На базе клинико-диагностического отделения № 1 Свердловского областного центра по профилактике и борьбе со СПИД и инфекционными заболеваниями был проведен ретроспективный анализ данных 87 пациентов с ВИЧ-инфекцией, находящихся под диспансерным наблюдением, получающих в течение не менее 12 мес. схему АРВТ, в состав которой был включен FPV/r.
Конечные точки: первичная конечная точка – определение доли пациентов с ВН < 400 копий/мл и < 150 копий/мл исходно и на 12-й, 24-й, 36-й и 48-й неделе исследования. Для количественной оценки уровня РНК ВИЧ в крови в исследовании использовали тест-системы с нижним порогом чувствительности 400, 150 и 50 копий/мл. Для удобства статистической обработки, пациенты, у которых использовали тест-системы с порогом чувствительности 150 и 50 копий/мл, были объединены. Вторичные конечные точки – изменение уровня СD4-лимфоцитов по сравнению с исходным на 12-й, 24-й, 36-й и 48-й неделе; изменение биохимических показателей крови (АлАТ, АсАТ, билирубин) и индекса APRI к 48-й неделе терапии.
Полученные результаты были обработаны статистически в программе Statistiсa 8 (фирмы StatSoft Inc., США) непараметрическими методами. Для парных сравнений в разных временных точках использовали критерий Вилкоксона, сравнение групп по качественному признаку проводили при помощи критерия соответствия χ2. Различия показателей признавали статистически достоверными при p < 0,05. Данные описывали с использованием медианы и квартилей (Me [Q25; Q75]).
Результаты и обсуждение
Общая характеристика пациентов
Были проанализированы данные 87 пациентов, принимающих FPV/r не менее 12 мес. У 54 (62%) пациентов были выявлены антитела к ВГС, у 3 (3,4%) – носительство HBsAg. Все пациенты получали FPV/r в комбинации с НИОТ. В качестве нуклеозидной основы большинство больных получали фиксированные комбинации доз (ФКД) ABC/3TC (кивекса) и ZDV/3TC (комбивир).
В зависимости от наличия или отсутствия ранее проводимой АРВТ больные были разделены на 2 группы. В 1-ю группу вошли 62 «наивных» пациента, которым FPV/r был назначен в схемах первой линии в дозе 1400/100 мг 1 раз в сутки. Во 2-ю группу вошли 25 пациентов, у которых была произведена замена ННИОТ на FPV/r в дозе 1400/100 мг 1 раз в сутки. У всех пациентов ВИЧ-инфекция находилась на стадии вторичных заболеваний. Группы были сопоставимы по возрасту, продолжительности заболевания, стадиям ВИЧ-инфекции. В качестве нуклеозидной основы в обеих группах более 50% больных получали ФКД ABC/3TC (кивекса). Значимые различия между группами отмечались в гендерном распределении (в 1-й группе достоверно больше было мужчин, во 2-й – женщин), исходных уровнях ВН (значимо ниже у пациентов 2-й группы; р = 0,001) и СD4-лимфоцитов (значимо выше у пациентов 2-й группы; р = 0,001). Основные исходные клинико-демографические характеристики групп представлены в табл. 1.
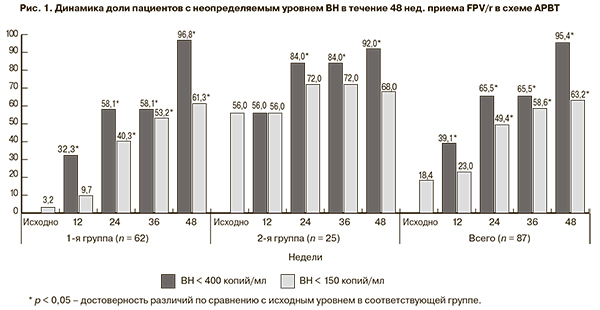
Оценка вирусологической эффективности
Полное подавление ВН к 48-й неделе терапии было достигнуто у 95,4% больных. Доля пациентов, достигших ВН < 400 копий/мл, к 24-й неделе АРВТ составила в 1-й группе 58,1%, во второй – 84%, а к 48-й неделе таких пациентов в 1-й группе было 96,8%, во 2-й – 92% (рис. 1). Доля пациентов, достигших ВН < 150 копий/мл, была больше во 2-й группе и уже к 24-й неделе составила 72% по сравнению с 40,3% в 1-й группе. К 48-й неделе терапии этого уровня достигли 61,3% пациентов 1-й группы и сохранили его 68% пациентов 2-й группы.
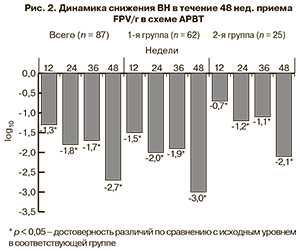
В общей группе больных наблюдалась положительная динамика снижения ВН. Так, к 12-й неделе она снизилась на 1,3 log10 (p = 0,0001), а к 48-й неделе – на 2,7 log10 (p = 0,0001; рис. 2). Следует отметить, что динамика снижения отмечалась в обеих группах. Медиана снижения в 1-й группе составила к 48-й неделе -3 log10 (p = 0,0001), во 2-й – - 2,1 log10 (p = 0,01).
Оценка иммунологической эффективности
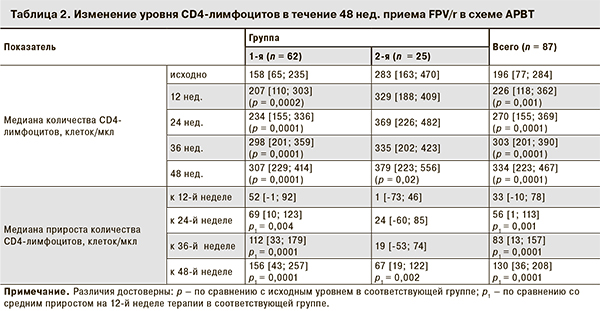
Показатели иммунного статуса у пациентов 1-й группы на момент начала лечения были существенно ниже, чем у пациентов 2-й группы, но к 48-й неделе АРВТ медиана уровня CD4-лимфоцитов в 1-й группе достигла 307 [229; 414] (р = 0,0001), во 2-й – 379 [223; 556] (р = 0,02; табл. 2).
Положительная динамика прироста CD4-лимфоцитов на протяжении 48 нед. терапии наблюдалась как в общей, так и в 1-й группе пациентов. Во 2-й группе отмечалось некоторое снижение медианы прироста CD4-лимфоцитов на 36-й неделе исследования, которое не показало статистической значимости. К 48-й неделе терапии наблюдался максимальный прирост во всех исследуемых группах, но более он был выражен в 1-й группе, где медиана прироста составила 156 клеток/мкл, тогда как во 2-й – 67 клеток/мкл (см. табл. 2).
Оценка гепатотоксичности
Анализ биохимических показателей крови показал, что после 48 нед. АРВТ с FPV/r в исследуемых группах не наблюдалось значительного повышения среднего уровня печеночных трансаминаз выше верхней границы нормы. Кроме того, отмечалось значимое снижение среднего уровня общего билирубина в общей группе пациентов, а также у больных 2-й группы (табл. 3).
Число пациентов, имеющих уровень биохимических показателей выше нормы на момент назначения схем АРВТ с FPV/r, к 48-й неделе лечения уменьшилось во всех группах, однако статистически значимым было только изменение числа пациентов с повышенным исходным уровнем АлАт (табл. 4).
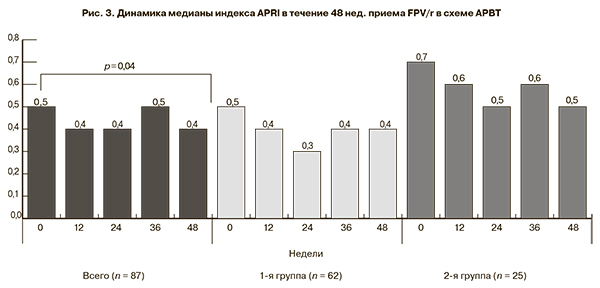
При оценке индекса APRI оказалось, что исходно его медиана не превышала верхнюю границу нормы (1,5) ни в одной группе и составила в общей группе 0,5 [0,3; 1,0], в 1-й – 0,5 [0,3; 0,7], во 2-й – 0,7 [0,4; 1,4] (р = 0,04 между 1-й и 2-й группами). На протяжении 48 нед. АРВТ с FPV/r не было повышения медианы индекса APRI ≥ 1,5 ни в одной из исследуемых групп (рис. 3).
Однако при индивидуальном анализе исходно были выявлены 15 пациентов с уровнем APRI ≥ 1,5 [min 1,7; max 367,8], через 48 нед. терапии их число уменьшилось до 10. Значения индекса APRI также снизились [min 2,1; max 60,9]. Высокий уровень индекса APRI объяснялся бóльшей лекарственной нагрузкой у пациентов с выраженным иммунодефицитом.
Обсуждение
Проведенное исследование показало высокую эффективность исследуемого препарата и безопасное влияние на функцию печени.
Основным определяющим критерием отбора пациентов в исследование явилась продолжительность лечения. Срок в 48 нед. с момента начала приема FPV/r мы посчитали достаточным для определения иммунологической и вирусологической эффективности препарата. Оценку гепатотоксичности мы провели, анализируя биохимические параметры крови (печеночные ферменты, билирубин) и индекс APRI .
Необходимо отметить, что к 48-й неделе лечения было достигнуто снижение ВН < 400 копий/мл у 95,4% исследуемых больных, < 150 копий/мл – у 63%, что характеризует FPV как антиретровирусный препарат с высокой вирусологической эффективностью и у «наивных» пациентов, и при замене ННИОТ. В 1-й группе ВН исходно была выше, при этом к 48-й неделе уровень < 400 копий/мл был достигнут у 96,8% пациентов. Полученные нами данные подтвердили результаты предыдущих исследований, свидетельствующие о высокой вирусологической эффективности схем АРВТ с FPV [2–6].
Положительная динамика прироста иммунного статуса наблюдалась у всех пациентов в течение 48 нед. Следует отметить, что в 1-й группе начало АРВТ было достаточно позднее, исходно медиана CD4-лимфоцитов составила 158 [65; 235] клеток/мкл. Медиана прироста в этой группе была максимальной и составила 156 [43; 257] клеток/мкл на 48-й неделе терапии. Во 2-й группе с исходно более высоким уровнем CD-лимфоцитов также отмечен прирост на протяжении всего периода наблюдения, однако максимальный прирост на 48-й неделе был ниже, чем в 1-й группе, и составил 67 [19; 122] клеток/мкл. Как правило, более высокая иммунологическая эффективность в группе «наивных» пациентов связана с исходно более низкими показателями иммунного статуса.
В общей группе пациентов (n = 87) все случаи высоких показателей печеночных ферментов объяснялись приемом совместно с АРВТ противотуберкулезных препаратов и наркотических веществ, что, к сожалению, часто отмечается в реальной практике. Несмотря на то, что из всех пациентов у 54 (62%) в крови были обнаружены антитела к ВГС, а трое (3,4%) являлись носителями HBsAg, в ходе анализа не было выявлено значимого повышения уровня печеночных ферментов в течение 48 нед., отмечалось даже некоторое снижение уровней АсАТ, общего и прямого билирубина. К 48-й неделе исследования достоверно уменьшилось число пациентов с исходно повышенным уровнем АлАт во всех группах. Также отмечено уменьшение числа пациентов с исходно повышенным уровнем АсАт и общего билирубина, однако статистическая значимость этих данных не подтвердилась. Возможно, снижение числа пациентов с исходно высоким уровнем трансаминаз и общего билирубина объясняется повышением иммунного статуса и отменой дополнительных лекарственных препаратов, назначенных с профилактической целью (отменяли ко-тримаксозол, флуконазол при повышении содержания CD4-лимфоцитов > 200 клеток/мкл и противотуберкулезные препараты – при завершении профилактического курса).
Длительный прием АРВТ, особенно у коинфицированных пациентов, получающих интегрированную терапию, повышает риск развития фиброза печени [7, 8]. При оценке динамики медианы индекса фиброза печени (APRI) в течение 48 нед. не отмечено её повышения > 1,5, что свидетельствует об отсутствии выраженного фиброза печени. Кроме того, отмечена даже тенденция к её снижению с 0,5 до 0,4 (p = 0,04) в общей группе пациентов. Полученные нами результаты согласуются с данными J. Pineda и соавт. [8] и косвенно подтверждают отсутствие влияния FPV на формирование фиброза печени у ВИЧ-инфицированных больных.
Таким образом, однократный прием FPV/r (1400/100 мг) в схемах АРВТ показал высокую иммунологическую и вирусологическую эффективность, отсутствие негативного влияния на функцию печени и хорошую переносимость у коинфицированных больных.


