Первые сообщения о новых представителях класса ненуклеозидных ингибиторов обратной транскриптазы (ННИОТ) стали появляться в 1990 г. Отличительной особенностью этой группы препаратов стало прямое блокирование участка обратной транскриптазы вируса иммунодефицита человека (ВИЧ), в результате чего замедлялся процесс полимеризации вируса.
На сегодняшний день в России зарегистрированы 4 представителя класса ННИОТ: невирапин (NVP), эфавиренз (EFV), этравирин (ETR) и рилпивирин (RPV). Препараты 1-го поколения – NVP и EFV – появились в 1996–1998 гг. Благодаря высокой эффективности, надежной фармакокинетике, удовлетворительной переносимости и простоте применения эти средства стали широко применять в схемах первой линии антиретровирусной терапии (АРВТ).
Однако возможность применения ННИОТ 1-го поколения в последующих линиях терапии была ограничена низким барьером развития генетической устойчивости к ВИЧ. Кроме этого, серьезные трудности вызывала перекрестная лекарственная устойчивость между NVP и EFV: было достаточно одной точечной мутации в положении 103 (K103N) гидрофобного участка связывания, чтобы исключить из арсенала АРВТ целую группу антиретровирусных препаратов (АРВП) [1].
 К представителям 2-го поколения ННИОТ относятся ETR (интеленс®) и RPV (эдюрант®). В июне 2008 г. в России был зарегистрирован ETR, который предназначен для лечения ВИЧ-инфекции, вызванной диким и устойчивым к другим ННИОТ и ингибиторам протеазы (ИП) штаммом ВИЧ-1, у взрослых больных, ранее получавших лечение [2].
К представителям 2-го поколения ННИОТ относятся ETR (интеленс®) и RPV (эдюрант®). В июне 2008 г. в России был зарегистрирован ETR, который предназначен для лечения ВИЧ-инфекции, вызванной диким и устойчивым к другим ННИОТ и ингибиторам протеазы (ИП) штаммом ВИЧ-1, у взрослых больных, ранее получавших лечение [2].
Эффективность ETR была продемонстрирована в исследованиях DUET-1 и DUET-2 в рамках III фазы клинических испытаний у больных с опытом терапии ВИЧ-инфекции и имевших 1 и более исходных мутаций резистентности ВИЧ к ННИОТ, а также 3 и более – к ИП. На протяжении 96 нед. применения ETR продемонстрировал высокую вирусологическую эффективность в сочетании с базовой АРВТ [дарунавир/ритонавир (DRV/r) 600/100 мг 2 раза в день и один выбранный исследователем нуклеозидный ингибитор обратной транскриптазы (НИОТ)] по сравнению с плацебо. Частота достижения неопределяемой вирусной нагрузки (ВН) была выше, чем в группе сравнения, независимо от исходного уровня РНК ВИЧ и содержания CD4+-клеток [2–7].
Спектр нежелательных явлений (НЯ) на фоне приема ETR и плацебо в течение 96 нед. терапии был сопоставим, за исключением частоты развития сыпи (21 и 12% соответственно). Серьезные НЯ встречались с одинаковой частотой (26% случаев). Различия лабораторных показателей крови (АлАТ, АсАТ, панкреатическая амилаза, липидный спектр) между группами также не отличались [8].
Результаты 48-недельного двойного слепого рандомизированного контролируемого исследования SENSE продемонстрировали достоверно меньшую частоту нейропсихических НЯ при использовании ETR в сравнении с EFV. Изменения показателей общего холестерина и липопротеидов низкой плотности были существеннее на фоне лечения EFV, чем ETR при сопоставимой вирусологической и иммунологической эффективности [9].
Наиболее трудно назначать лечение пациентам, длительно получавшим АРВТ и имевшим в анамнезе вирусологические или иммунологические неудачи. Для этих пациентов с множественной резистентностью было проведено исследование TRIO, в котором была продемонстрирована эффективность комбинации трех новых АРВП: ингибитора интегразы ралтегравира (RAL), ННИОТ (ETR) и ИП DRV/r. Предложенный режим лечения показал высокую эффективность, сравнимую с эффективностью АРВТ у больных, впервые получающих терапию (неопределяемая ВН на 24-й и 48-й неделях лечения составила 90 и 86% соответственно). Кроме этого, терапия относительно хорошо переносилась [10].
Цель нашего исследования – оценить эффективность и безопасность использования ETR в составе комбинированной АРВТ ВИЧ-инфекции у пациентов с неэффективностью или непереносимостью схем, включающих ННИОТ 1-го поколения или ИП.
Материалы и методы
На сегодняшний день у нас уже есть собственный опыт применения этого препарата. В Ленинградском областном Центре по профилактике и борьбе со СПИД и инфекционными заболеваниями под наблюдением находились 10 пациентов, у которых в схеме лечения применялся препарат ETR после вирусологической неудачи или выявления НЯ в схемах терапии с ННИОТ 1-го поколения и ИП. Диагноз и стадия заболевания пациентам были установлены на основании клинико-лабораторных и эпидемиологических данных. Схемы АРВТ подбирали с учетом возраста, лабораторных показателей, сопутствующей патологии и возможных лекарственных взаимодействий. Для статистического анализ полученных данных применяли непараметрические методы (критерии различия для зависимых выборок) с использованием статистического пакета SPSS.17.0
 Среди пациентов было 7 мужчин и 3 женщины в возрасте от 27 до 53 лет (средний возраст – 37,2 года). По данным анамнеза у 5 больных установлен половой путь инфицирования и у 5 – парентеральный, через инъекционное потребление наркотических средств. Злоупотребление алкоголем на момент анализа отметили 3 пациента.
Среди пациентов было 7 мужчин и 3 женщины в возрасте от 27 до 53 лет (средний возраст – 37,2 года). По данным анамнеза у 5 больных установлен половой путь инфицирования и у 5 – парентеральный, через инъекционное потребление наркотических средств. Злоупотребление алкоголем на момент анализа отметили 3 пациента.
Продолжительность ВИЧ-инфекции составила от 2 до 11 лет (в среднем 7,6 + 0,9 года). При включении в исследование были установлены следующие стадии ВИЧ-инфекции: 4А – у 8 пациентов, 4Б – у 1, 4В – у 1.
У всех пациентов регистрировали клинические проявления вторичных заболеваний, в том числе у 1 пациента с проявлением СПИДа в виде кандидозного эзофагита и генерализованного туберкулеза (поражение ЦНС, периферических лимфатических узлов, органов дыхания в виде диссеминированного туберкулеза легких).
Выявлена следующая сопутствующая патология: в 7 случаях – хронический гепатит С, в том числе в 2 – микст-инфекция В + С, по 1 случаю сахарного диабета, бронхиальной астмы, аутоиммунного тиреоидита, энцефалопатии смешанного генеза.
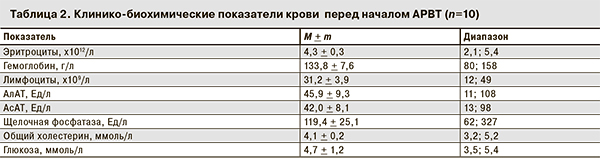
Количество CD4-лимфоцитов перед назначением первой схемы лечения колебалось от 106 до 305 клеток/мкл и составило в среднем 203 + 24,2 клетки/мкл, при этом показатель менее 200 клеток/мкл был зарегистрирован у 7 из 10 пациентов. Уровень РНК ВИЧ на старте АРВТ определялся у всех пациентов, в среднем он составил 383 797 + 166 721 копий/мл (табл. 1). ВН более 100 000 копий/мл была у 8 человек.
При оценке показателей гемограммы и биохимического исследования крови перед стартом АРВТ были выявлены следующие изменения:
– анемия (снижение эритроцитов и гемоглобина) – у 1 женщины и 1 мужчины;
– низкое содержание лимфоцитов – у 1 женщины (15 х 109/л) и 3 мужчин (12–19 х 109/л);
– повышение уровня трансаминаз печени более 40 Ед/л (от 69 до 108 Ед/л) – у 3 человек, имевших сопутствующие хронические гепатиты С и С + В;
– повышение уровня щелочной фосфатазы – у 1 пациентки с хроническим гепатитом С.
Показатели липидного профиля и углеводного обмена соответствовали норме (табл. 2).
Всем пациентам была назначена схема лечения в соответствии со стандартами лечения больных ВИЧ-инфекцией. В 6 случаях она включала зидовудин/ламивудин (ZDV/3TC) + EFV. Кроме того, учитывая изменения лабораторных показателей, сопутствующую патологию и возраст, были использованы и другие комбинации АРВП. Так, остальные 4 пациента начинали лечение по следующим схемам: зидовудин/ламивудин (AZT/3TC) + лопинавир/ритонавир (LPV/r) (1); AZT/3TC + атазанавир/ритонавир (ATV/r) (1); абакавир (ABC) + 3TC + LPV/r (1); ставудин (d4T) + 3TC + EFV (1).
Длительность АРВТ первой линии составила от 2 до 51 мес. (в среднем 18,4 + 5,3 мес.), при этом терапию менее 1 года получали 5 больных, двое пациентов продолжали терапию первой линии более 3 лет (3,5 и 4,3 года соответственно).

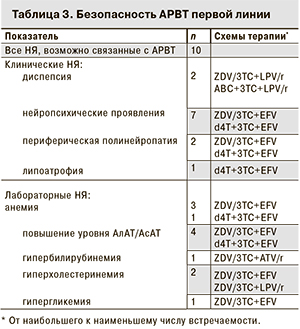
В процессе лечения среднее количество СD4-лимфоцитов составило 242,8 + 58,8 клетки/мкл, уровень менее 200 клеток/мкл сохранялся у 1 пациента, более 350 клеток/мкл – у 3. Уровень ВН был неопределяемым у 9 больных, продолжающих лечение, у 1 пациента уровень РНК ВИЧ составил 405 059 копий/мл. При проведении теста у него была выявлена резистентность ко всем препаратам класса НИОТ и ННИОТ 1-го поколения. Все пациенты имели клинические проявления НЯ различной степени тяжести, возможно, связанные с приемом АРВП. Чаще всего (у 7 пациентов) отмечали нейропсихические нарушения, связанные с приемом EFV; дисфункцию желудочно-кишечного тракта отмечали у 2 пациентов; в 2 случаях имела место периферическая полинейропатия, в 1 – липоатрофия. Изменение лабораторных показателей чаще всего было связано с повышением уровня печеночных ферментов (у 4 больных). Развитие анемии до 2-й степени тяжести отмечено в 4 случаях, гиперхолестеринемия – в 2, гипербилирубинемия – в 1 и гипергликемия – в 1 (табл. 3).
У всех больных были изменены схемы АРВТ в соответствии с возникшими НЯ: 6 пациентам была изменена нуклеозидная основа (AZT/3TC заменен на ABC + 3TC), а в 3 случаях в схеме лечения продолжали использовать AZT/3TC. Одному пациенту схемы лечения неоднократно изменяли в противотуберкулезной больнице. В конечном итоге, на момент исследования, он продолжал лечение по схеме диданозин (ddI)+ABC+RAL. Однако у 6 пациентов по-прежнему отмечали нейропсихические расстройства различной степени тяжести, у 2 была дисфункция желудочно-кишечного тракта, у 1 – гипербилирубинемия. Следует отметить, что развитие НЯ крайне негативно сказывается на приверженности пациентов к лечению и в конечном итоге приводит к срыву и отказу от лечения.
Целью включения в схему лечения ETR было как улучшение переносимости и повышение эффективности АРВТ, так и усиление влияния на приверженность пациентов к лечению. Лишь в одном случае изменение схемы было показано с учетом подтвержденной резистентности ко всем препаратам класса НИОТ и ННИОТ 1-го поколения и выявленной чувствительности ВИЧ к ETR, DRV/r и энфувиртиду (ENF).
ETR назначали согластно инструкции по медицинскому применению в дозе 200 мг (2 таблетки х 100 мг) 2 раза в день после еды [11].
Перед переходом на прием ETR пациенты получали следующие комбинации АРВП: ZDV/3TC + EFV (3), ABC + 3TC + EFV (3), ABC + 3TC + ATV/r (1), ABC + 3TC + LPV/r (2) и ABC + ddI + RAL (1).
Следует отметить, что на данном этапе у 9 пациентов в схемах лечения была сохранена нуклеозидная основа. Только 1 пациенту была проведена полная замена схемы АРВТ после получения результата теста на резистентность. Лечение было продолжено по схеме: ETR + DRV/r (1200/200 мг/сутки) + ENF.
На момент проведения исследования продолжительность приема ETR составила от 1 до 22 мес. Лишь в одном случае не был достигнут неопределяемый уровень ВН, что, по всей видимости, было связано с кратковременностью прима ETR и изначально высоким уровнем ВН. Следует отметить, что в процессе наблюдения все пациенты имели неопределяемый уровень ВН. По сравнению с ранее использованными схемами лечения средний уровень СД4-лимфоцитов составил 361 + 53,6 клетки/мкл, что подтверждает высокую иммунологическую активность препарата (табл. 4).
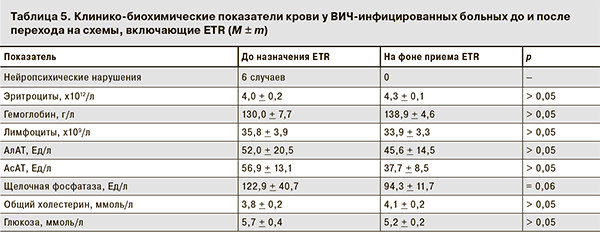
Следует отметить, что во всех случаях сохранялись стабильными показатели гемограммы, значительно улучшились биохимические показатели крови, изменений углеводного и липидного обмена выявлено не было (табл. 5). Все пациенты отметили отсутствие нейропсихических НЯ при использовании ETR по сравнению с EFV.
Таким образом, наш опыт применения ETR в суточной дозе 400 мг подтвердил высокий терапевтический эффект препарата у пациентов с разным анамнезом применения АРВП. Схемы терапии, в состав которых входил ETR, отличались высокой эффективностью и благоприятным профилем безопасности. За время наблюдения НЯ зарегистрировано не было, что, безусловно, является положительным фактором для формирования и поддержания приверженности к лечению.


