Воспаление является ключевым механизмом в патогенезе новой коронавирусной инфекции COVID-19. Прогрессирование воспалительных реакций приводит к развитию пневмонии, цитокинового шторма, острого респираторного дистресс-синдрома и летального исхода [1, 2]. В связи с этим возрастает роль упреждающей противовоспалительной терапии (УПТ), целью которой является предотвращение или купирование симптомокомплекса гипервоспаления.
Согласно Временным методическим рекомендациям «Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19)» [3], в качестве УПТ следует использовать ингибиторы янус-киназ или рецепторов интерлейкинов 1 и 6 (ИЛ-1 и ИЛ-6), ингибиторы самого ИЛ-6, а также системные глюкокортикостероиды.
Олокизумаб (Артлегиа) представляет собой гуманизированное моноклональное антитело, которое связывается с ИЛ-6, нейтрализуя его эффекты [4]. Препарат доказал свою эффективность и безопасность в терапии пациентов с ревматоидным артритом [5], а также в качестве патогенетической терапии COVID-19.
Он рекомендован для лечения пациентов с легким течением и наличием факторов риска тяжелого течения COVID-19 в дозе 64 мг подкожно (п/к), для пациентов со среднетяжелым течением – в дозе 64–128 мг внутривенно (в/в), при тяжелом или крайне тяжелом течении – в дозе 128 мг в/в. Препарат вводят однократно, при недостаточном эффекте возможно повторное введение.
Достижение максимальной концентрации препарата при введении в/в происходит значительно быстрее, чем при введении п/к. В ходе открытого многоцентрового рандомизированного клинического исследования RESET (CL04041094) определяли фармакокинетические параметры олокизумаба при однократном введении в/в пациентам с COVID-19 [6]. Медиана (Ме) Tmax (время достижения максимальной концентрации) при введении в/в дозы 128 мг составила 6 [2; 18] ч. В исследованиях с участием пациентов с ревматоидным артритом при введении в/в в дозе 1 мг/ кг Ме Tmax олокизумаба составила 2 [2; 14] ч соответственно. В то же время при введении препарата п/к пациентам с ревматоидным артритом в дозах 1 и 3 мг/ кг Ме Tmax составили 313 и 171 ч соответственно. Таким образом, с учетом высокой скорости патогенетических процессов, возникающих при COVID-19, введение олокизумаба в/в может способствовать более благоприятному прогнозу.
Материалы и методы
RESMICA (CL04041178) представляет собой многоцентровое неинтервенционное ретроспективное исследование, целью которого является определение профиля пациентов и оценка эффективности применения различных доз и способов введения препарата олокизумаб у пациентов с COVID-19 легкого и среднетяжелого течения.
Исследование проводили в период с января 2021 г. по октябрь 2022 г. На протяжении этого периода в клинических центрах не отмечалось значимых проблем с доступностью препарата, а врачи имели возможность свободно назначать олокизумаб в любой из рекомендованных доз. Около 25% пациентов, данные которых были использованы для анализа, проходили лечение COVID-19 в период с января 2021 г. по январь 2022 г., когда доминирующим вариантом SARS-CoV-2 на территории России был дельта (B.1.617.2). Остальные пациенты были госпитализированы в период, когда доминирующим вариантом SARS-CoV-2 стал омикрон, который характеризуется значительно более низкой летальностью и более высокой контагиозностью по сравнению с вариантом дельта. Никаких дополнительных лечебных и диагностических процедур пациентам в рамках исследования не проводилось. Сбор данных осуществляли анонимно ретроспективно, в связи с чем подписание информированного согласия пациентами не требовалось.
Протокол исследования RESMICA был рассмотрен и одобрен Независимым междисциплинарным Комитетом по этической экспертизе клинических исследований, а также локальными этическими комитетами при исследовательских центрах. Исследование проводилось на базе 14 клинических центров в Российской Федерации.
Изучали медицинские данные пациентов, собранные в начале госпитализации, в день начала терапии (исходная точка), а также на протяжении госпитализации в дни 2 ± 1, 4 ± 1, 7 ± 1, 10 ± 1, 14 ± 1, 28 ± 1 от начала терапии олокизумабом. В качестве данных об исходной точке могла быть использована информация из точки «начало госпитализации», если терапию начинали в день госпитализации или на следующий день. Анализировали анамнез основного заболевания, демографические и антропометрические характеристики, данные о сопутствующих заболеваниях и их терапии, результаты лабораторных обследований, сатурацию, температуру тела, результаты компьютерной томографии (КТ), информацию о назначении кислородной поддержки, переводе в отделение реанимации и интенсивной терапии (ОРИТ) и других значимых медицинских событиях.
В исследовании участвовали лица старше 18 лет с подтвержденным диагнозом COVID-19 легкого или среднетяжелого течения, которые были госпитализированы и получали терапию олокизумабом в одном из режимов дозирования: 64 мг п/к, 128 мг п/к, 64 мг в/в или 128 мг в/в.
Критерии включения: наличие патологических изменений в легких, соответствующих КТ-1 или КТ-2, в сочетании с двумя и более признаками: SpO2 – 94% и выше; уровень С-реактивного белка (СРБ) в пределах 3–9 ВГН (верхних границ нормы); температура тела 37,5 оС и выше в течение 3–5 дней; лейкоциты – 3,0–4,0×109/л, лимфоциты – 1,0–2,0×109/л.
Критерии невключения: наличие признаков тяжелого и крайне тяжелого течения COVID-19; перевод пациента в ОРИТ до назначения олокизумаба; применение до начала терапии олокизумабом других препаратов УПТ, а также высоких доз глюкокортикостероидов.
Выбор дозы и способа введения олокизумаба врачи осуществляли на основании клинических и лабораторных признаков COVID-19.
Эффективность оценивали по следующим конечным точкам: частоте летальных исходов; суммарной частоте перевода в ОРИТ на неинвазивную или инвазивную вентиляцию легких (НИВЛ/ИВЛ) и/или летальных исходов; динамике лабораторных показателей в течение исследования; частоте клинического улучшения или ухудшения, оцениваемой по 11-категориальной шкале клинического прогрессирования ВОЗ, согласно которой:
0 – неинфицированный; отсутствие вирусной РНК при проведении ПЦР на SARS-CoV-2;
1 – амбулаторный; отсутствие клинических проявлений, определяется РНК вируса SARS-CoV-2;
2 – амбулаторный; есть клинические проявления, нет ограничений активности;
3 – амбулаторный; есть клинические проявления, есть ограничения активности (нуждается в помощи);
4 – госпитализирован; кислородная поддержка не требуется;
5 – госпитализирован; требуется кислородная поддержка (маска или носовые канюли);
6 – госпитализирован; НИВЛ или высокопоточная оксигенация;
7 – интубация; ИВЛ (индекс оксигенации pO2/FiO2 ≥ 150 или SpO2/FiO2 ≥ 200);
8 – ИВЛ (индекс оксигенации pO2/FiO2 < 150 или SpO2/FiO2 < 200) или вазопрессоры;
9 – ИВЛ, (индекс оксигенации pO2/FiO2 < 150) и вазопрессоры, диализ или ЭКМО;
10 – летальный исход.
Под клиническим улучшением понимали снижение оценки по крайней мере на 1 балл по сравнению с исходным значением. Оценку проводили на день 7 ± 1 после введения олокизумаба.
Клиническим ухудшением считали повышение оценки по меньшей мере на 1 балл по сравнению с исходным. Оценку производили за весь период наблюдения.
Уровень статистической значимости оценки различий принят равным 0,05. С учетом многогруппового характера сравнений была применена поправка на ошибку 1-го рода по методу Бонферрони. Различия центральных тенденций оценивали с помощью теста Манна–Уитни. Для оценки различий категориальных данных использован точный тест Фишера. Линейную связь между переменными оценивали с помощью коэффициентов корреляции Спирмена.
Для оценки влияния независимых переменных на конечные точки построена обобщенная линейная модель с бинарным откликом, где все демографические, лабораторные и инструментальные данные, а также все факторы риска тяжелого течения заболевания учтены в качестве фиксированных эффектов. Подбор наилучшей модели осуществляли путем пошагового включения/исключения потенциальных предикторов и их взаимодействий с максимизацией С-статистики (площади под ROC-кривой) и минимизацией информационного критерия Акаике (AIC), контроль мультиколлинеарности – путем проверки коэффициентов инфляции дисперсии.
Результаты
В исследование были включены 1104 пациента. В зависимости от дозы и способа введения олокизумаба они были разделены на 4 группы: 64 мг п/к получали 363 чел. (1-я группа), 64 мг в/в – 210 чел. (2-я группа), 128 мг п/к – 210 чел. (3-я группа) и 128 мг в/в – 321 чел. (4-я группа).
При оценке исходных характеристик группы сравнивали попарно. Было сформировано 4 пары: 1-я и 2-я группы (64 мг п/к и в/в); 3-я и 4-я (128 мг п/к и в/в); 1-я и 3-я (64 и 128 мг п/к); 2-я и 4-я (64 и 128 мг в/в)
Исходные данные пациентов представлены в табл. 1
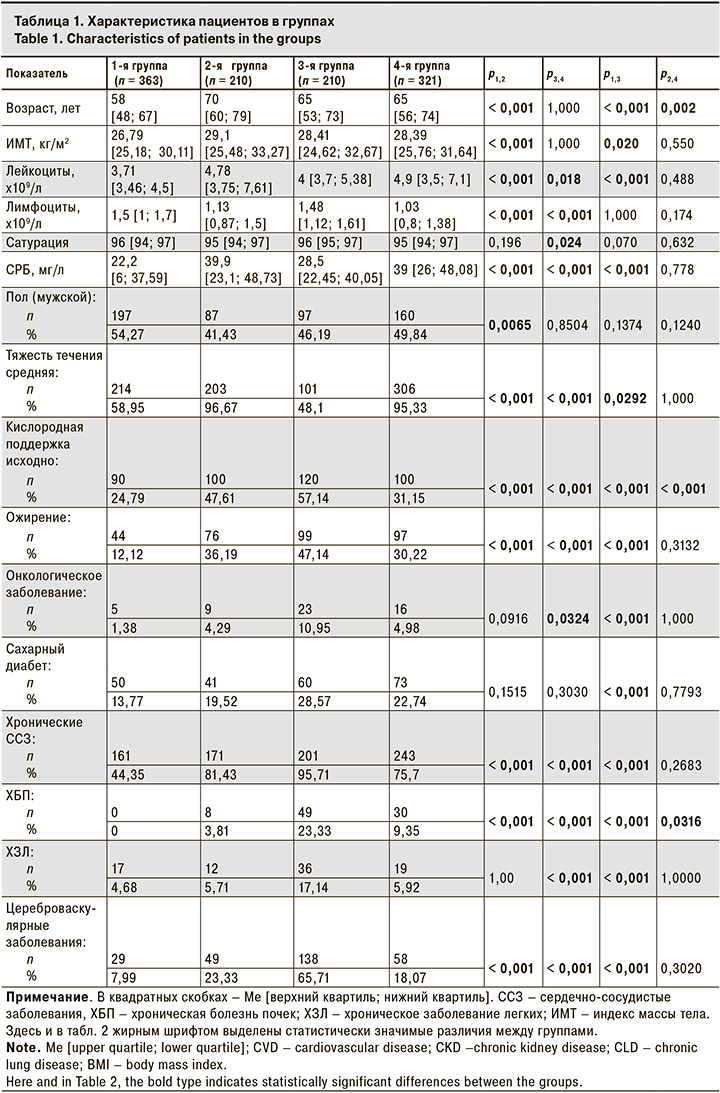
В 1-й группе (64 мг п/к) у 41% участников исходная тяжесть течения COVID-19 оценивалась как легкая. Большинство пациентов на момент начала терапии олокизумабом не получали кислородной поддержки. Ме уровня СРБ в исходной точке составила 22,2 [6; 37,59] мг/л, а Ме количества лимфоцитов – 1,5 [1; 1,7] х109/л. По крайней мере одно заболевание, относящееся к факторам риска развития тяжелого течения COVID-19, имели 190 (52,3%) чел., 165 (45,5%) чел. были в возрасте 60 лет или старше.
В 3-й группе (128 мг п/к) 51,9% пациентов до начала противовоспалительной терапии имели легкую степень тяжести заболевания, при этом кислородная поддержка была назначена 120 (57,14%) из них. Ме уровня СРБ в исходной точке составила 28,5 [22,45; 40,05] мг/л, а Ме количества лимфоцитов – 1,48 [1,12; 1,61] х109/л У пациентов этой группы отмечена достаточно высокая коморбидность: в среднем на каждого пациента приходилось по 4,26 заболевания из числа факторов риска тяжелого течения COVID-19. 136 (64,8%) чел. были в возрасте 60 лет и старше. Значения лабораторных показателей в этой группе соответствовали клинической картине легкого и среднетяжелого течения заболевания.
По многим исходным параметрам разница между пациентами 2-й (64 мг в/в) и 4-й (128 мг в/в) групп не имела статистической значимости. Среднетяжелое течение COVID-19 на момент начала терапии наблюдалось более чем у 95% пациентов. Ме уровней СРБ составили 39,9 [23,1; 48,73] и 39 [26; 48,08] мг/л соответственно. Более 65% пациентов, получавших олокизумаб в/в, имели исходный уровень СРБ, равный 6 ВГН (30 мг/л) и выше. У большинства пациентов в этих группах наблюдалась выраженная лимфопения. Ме количества лимфоцитов во 2-й группе составила 1,13 [0,87; 1,5] х109/л, в 4-й группе –1,03 [0,8; 1,38] х 109/л. В обеих группах каждый пациент в среднем имел по 2 сопутствующих заболевания, относящихся к факторам риска тяжелого течения COVID-19.
Наиболее значимые с клинической точки зрения различия наблюдались при разных способах введения препарата. Пациенты 2-й и 4-й групп имели исходно более высокий уровень СРБ и более низкий уровень лимфоцитов по сравнению с пациентами 1-й и 3-й групп, что свидетельствует о более выраженной воспалительной реакции. Более чем у 95% больных 2-й и 4-й групп течение COVID-19 было среднетяжелым, в то время как в 1-й и 3-й группах среднетяжелое течение отмечали у 214 (58,95%) и 101 (48,1%) пациента.
Возраст и ИМТ пациентов во 2-й группе были значительно выше, чем в 1-й. Частота всех оцениваемых сопутствующих заболеваний, кроме ХЗЛ, во 2-й группе также была выше.
Примечательно, что самая высокая частота сопутствующих заболеваний, относящихся к факторам риска, была у пациентов 3-й группы. Так, 95,71% из них имели хроническое ССЗ, в то время как в 4-й группе этот показатель составил 75,7% (р < 0,001). Ожирение наблюдалось в этих группах у 47,14 и 30,22% пациентов соответственно (р < 0,001). Таким образом, в 3-й группе риск развития тяжелого течения COVID-19 был выше, чем в 4-й, однако данные клинико-лабораторного обследования показали, что исходно пациенты 4-й группы имели более выраженные признаки воспаления. В частности, более высокий уровень СРБ, и более низкие уровни сатурации и лимфоцитов говорят о более тяжелом состоянии пациентов 4-й группы. Это наблюдение подтверждается тем, что в 4-й группе гораздо больше пациентов имели исходно среднетяжелое течение COVID-19, чем в 3-й группе (р < 0,001).
Пациенты 2-й и 4-й групп не имели статистически значимой разницы по большинству оцениваемых показателей. Частота сопутствующих заболеваний, которые относятся к факторам риска тяжелого течения COVID-19, в этих группах была сопоставимой. Статистически значимые различия между этими группами наблюдались только по частоте назначения кислородной поддержки в исходной точке, возрасту и частоте ХБП, при этом Ме возраста пациентов во 2-й группе была выше, чем в 4-й – 70 [60; 79] и 65 [56; 74] лет соответственно (р = 0,001).
Таким образом, пациентам с более выраженными признаками COVID-19 чаще назначали олокизумаб в/в. Различия между пациентами, получавшими разные дозы препарата в рамках одного способа введения были менее выражены.
Результаты оценки основных конечных точек представлены в табл. 2.
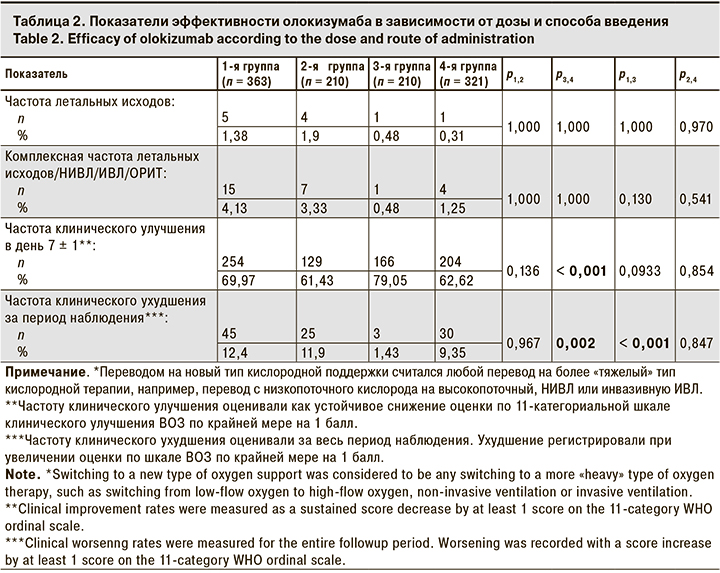
Было зарегистрировано всего 11 летальных исходов: 5 в 1-й группе, 4 – во 2-й, по 1 – в 3-й и 4-й группах. Из-за небольшого числа летальных исходов невозможно было провести полноценную статистическую оценку этого показателя. Все пациенты с летальным исходом имели по крайней мере 1 фактор риска развития тяжелого течения COVID-19, 10 из них имели 2 и более заболевания, относящихся к факторам риска. Исходная тяжесть течения заболевания у этих пациентов оценивалась как среднетяжелая, а сатурация не превышала 95%. У 9 пациентов с летальным исходом уровень СРБ в исходной точке превышал 6 ВГН (> 30 мг/л). 4 из 11 пациентов с летальным исходом были госпитализированы до февраля 2022 г., когда доминировал вариант SARS-CoV-2 дельта, который характеризуется более высокой летальностью. Частота летальных исходов составила 1,5% (4/274). Среди пациентов, госпитализированных в период доминирования варианта омикрон, этот показатель был существенно ниже – 0,8% (7/830).
Оценивали комплексную частоту переводов на НИВЛ или ИВЛ, в ОРИТ или летальных исходов. В 1-й группе они были зарегистрированы у 15 (4,13 %) пациентов, в 4-й группе – у 4 (1,25%), в 3-й – у 1 (0,48%). При сравнении групп попарно статистически значимой разницы не выявлено. У всех пациентов, которым потребовался перевод на НИВЛ/ИВЛ, в ОРИТ или у которых был зарегистрирован летальный исход, исходно течение заболевания было оценено как среднетяжелое.
Частоту клинического улучшения оценивали по шкале ВОЗ в день 7 ± 1 после введения олокизумаба. Она была выше при введении п/к, однако статистически значимую разницу наблюдали только при сравнении 3-й и 4-й групп. Клиническое улучшение наблюдалось у 166 (79%) и 201 (62,6%) пациентов в 3-й и 4-й группах соответственно (р < 0,001).
Статистически значимая разница по частоте клинического ухудшения, согласно шкале ВОЗ, была отмечена между 3-й и 4-й группами: в 3-й группе показатель был ниже (1,43%), чем в 4-й (9,35%) (p = 0,002). Различия были значимы также при сравнении 1-й и 3-й групп: в 1-й группе показатель был на 10% выше (p = 0,001).
На динамику заболевания и частоту наступления исходов могли оказать значимое влияние факторы, связанные с исходной тяжестью течения заболевания или наличием у пациентов факторов риска. Для оценки влияния таких факторов на основные конечные точки «частота НИВЛ/ИВЛ/ОРИТ/летальных исходов», «частота клинического улучшения в день 7 ± 1», «частота клинического ухудшения за весь период» были построены соответствующие линейные модели.
Для всех моделей в первичный анализ включали следующие предикторы: возраст; пол; ИМТ; уровень СРБ, лейкоцитов, лимфоцитов, сатурации в исходной точке; тяжесть течения; доза и способ введения олокизумаба; наличие кислородной поддержки исходно; исходный объем поражения легких по КТ; наличие отдельных заболеваний, относящихся к факторам риска развития тяжелого течения (сахарный диабет, хронические ССЗ и т. д.). Факторы, которые при первичном анализе оказались значимы на уровне 0,1, далее использовали для подбора наилучшей модели.
Финальные модели представлены на рис. 1.

Ключевыми факторами, увеличивающими вероятность перевода на НИВЛ и ИВЛ, в ОРИТ или наступления летального исхода, являются возраст старше 60 лет, сниженная сатурация и наличие у пациента выраженных признаков поражения легких по КТ. Повышение дозы олокизумаба до 128 мг и более раннее назначение кислородной поддержки снижают вероятность наступления этих событий, в частности, увеличение дозы снижает шансы этих событий более чем в 6 раз (ОШ 0,15; 95% ДИ 0,04–0,42).
Значимыми факторами, снижающими шансы на клиническое улучшение в день 7, были возраст старше 60 лет, повышенный уровень лейкоцитов и ожирение. У пациентов, которым исходно назначали кислородную поддержку, шансы на клиническое улучшение были выше. Доза и способ введения олокизумаба не были значимы в данной модели.
Увеличивают шансы клинического ухудшения ожирение, исходное среднетяжелое течение COVID-19, наличие поражения легких, соответствующее КТ-2 по сравнению с КТ-1, а также наличие цереброваскулярных заболеваний. Введение олокизумаба в дозе 128 мг позволяет снизить шансы клинического ухудшения почти в 3 раза (ОШ 0,34; 95% ДИ 0,2–0,56). Кроме того, применение кислородной терапии в исходной точке также способствует снижению шансов клинического ухудшения.
Учитывая статистически значимое влияние дозы препарата на вероятность исходов в двух исследованных моделях, можно сделать вывод о целесообразности применения более высокой дозы олокизумаба (в настоящем исследовании 128 мг) у пациентов с признаками среднетяжелого течения COVID-19.
В рамках исследования была оценена динамика основных лабораторных маркеров, отражающих тяжесть течения COVID-19: СРБ и лимфоцитов (рис. 2).
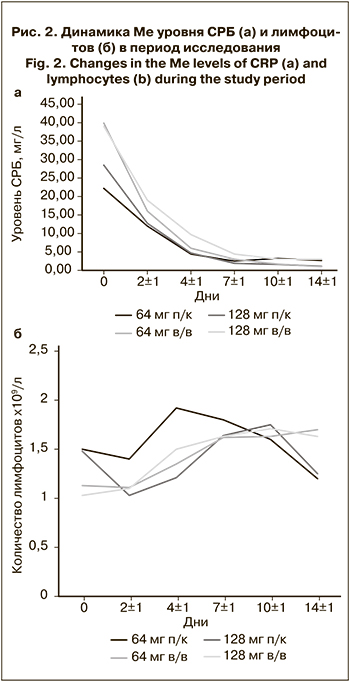
В целом с первых дней исследования наблюдалась положительная тенденция к снижению уровня СРБ. Уже на 2-й день после начала терапии он снизился почти вдвое во всех группах. Cнижение Ме уровня СРБ через 2 дня после введения олокизумаба составило в 1-й группе 45,94% (10,2 мг/л) , во 2-й – 59,8% (23,84 мг/л), в 3-й – 55,26% (15,75 мг/л) и в 4-й – 51,28% (20 мг/л).
При п/к ведении препарата Ме уровня СРБ достигала нормальных значений (< 5 мг/л) уже в день 4 ± 1, а при введении в/в – в день 7 ± 1, однако следует отметить, что исходный уровень СРБ у пациентов 2-й и 4-й групп был значительно выше, чем у остальных.
Динамика количества лимфоцитов также имела благоприятную тенденцию. Начиная с дня 4 ± 1 после введения олокизумаба их уровень увеличивался во всех группах. Примечательно, что в день 2 ± 1 в 1-й и 3-й группах Ме количества лимфоцитов снизилась относительно исходного уровня, в то время как во 2-й и 4-й группах показатель оставался на уровне исходных значений или даже увеличивался. Такая особенность может быть связана с различиями в фармакокинетике олокизумаба в зависимости от способа его введения. Поскольку время достижения максимальной концентрации препарата после введения в/в значительно ниже, можно предположить, что эффект терапии при этом развивался быстрее, препятствуя снижению уровня лимфоцитов.
Обсуждение
Так как исследование имеет неинтервенционный ретроспективный дизайн, при интерпретации результатов следует учитывать некоторые ограничения.
Для анализа были доступны только ретроспективные медицинские данные пациентов, собранные в рамках рутинной клинической практики, в связи с этим некоторые данные отсутствовали и не подлежали замене или восстановлению.
В исследовании также не была предусмотрена рандомизация пациентов, поэтому сравниваемые группы значимо различались по некоторым демографическим и исходным характеристикам. Поскольку одной из целей исследования была оценка исходного профиля пациентов, которым назначают разные дозы олокизумаба, такие отличия являлись ожидаемыми и приемлемыми. Однако следует учитывать, что на основании полученных в данном анализе показателей эффективности невозможно сделать однозначные выводы о преимуществах того или иного режима назначения препарата.
На оценку конечных точек эффективности также могли влиять различные неучтенные факторы, например, различия в подходах к терапии и обследованию пациентов в медицинских учреждениях, участвовавших в исследовании. Кроме того, за период исследования произошла смена доминирующего штамма SARS-CoV-2. Как известно, вариант омикрон, который стал доминирующим в России в начале 2022 г., связан с более легким течением COVID-19 по сравнению со штаммом дельта, который доминировал в 2021 г. Поскольку определение варианта вируса проводится не всем пациентам, оценить влияние штамма на показатели эффективности олокизумаба не представлялось возможным.
В рамках исследования сравнивали 4 группы пациентов, получавших олокизумаб в дозах 64 или 128 мг п/к или в/в.
Лабораторные показатели в целом соответствовали критериям назначения УПТ у пациентов с легким или среднетяжелым течением COVID-19. Пациенты имели невысокий уровень коморбидности. В ходе оценки эффективности было выявлено, что среди 363 пациентов 1-й группы, которым был назначен олокизумаб в дозе 64 мг п/к, у 15 (4,13 %) наблюдалось клиническое ухудшение, которое потребовало назначения НИВЛ/ИВЛ, перевода в ОРИТ или привело к летальному исходу. Все пациенты, у которых были зарегистрированы эти неблагоприятные явления, имели исходно среднетяжелое течение COVID-19, у 12 из них исходный уровень СРБ был выше 6 ВГН. Это свидетельствует о целесообразности назначения более высокой дозы и/или в/в введения олокизумаба пациентам со среднетяжелым течением с учетом критериев назначения УПТ [3]. Доза 64 мг п/к рекомендована пациентам с легким течением заболевания и наличием факторов риска тяжелого течения.
Олокизумаб в дозе 128 мг п/к назначали пациентам 3-й группы с легким и среднетяжелым течением заболевания, соотношение которых составило примерно 1:1, и более высоким уровнем коморбидности. Показатели эффективности в этой группе свидетельствуют о положительном влиянии такого режима применения препарата на клиническую динамику пациентов с COVID-19.
Практически все пациенты, которым было назначено в/в введение олокизумаба, имели среднетяжелое течение COVID-19. Уровень СРБ у многих из них превышал 6 ВГН. Большинство имели одновременно несколько заболеваний, относящихся к факторам риска тяжелого течения, а также возраст старше 60 лет. При введении препарата в/в наблюдалась низкая частота неблагоприятных явлений, что подтверждает целесообразность назначения олокизумаба в/в пациентам со среднетяжелым течением COVID-19.
Отдельно следует отметить результаты моделирования, выполненного на основании включенных в исследование данных. Ключевыми факторами, увеличивающими вероятность неблагоприятного течения заболевания, являлись возраст старше 60 лет, сниженная сатурация кислорода и наличие у пациента выраженных признаков поражения легких по КТ, что соответствует существующим представлениям о факторах риска и основным характеристикам тяжести течения заболевания. Назначение олокизумаба в дозе 128 мг и более раннее назначение кислородной поддержки снижают вероятность неблагоприятного течения COVID- 19.
Результаты анализа динамики лабораторных показателей также отражают патогенетическое воздействие олокизумаба на процессы гипервоспаления, что представлено снижением уровня СРБ и восстановлением уровня лимфоцитов после начала терапии. Снижение Ме количества лимфоцитов в день 2 ± 1 у пациентов, получавших олокизумаб п/к, и отсутствие его при введении в/в согласуется с данными других исследователей [7, 8].
Заключение
Таким образом, полученные результаты соответствуют сложившимся к настоящему дню представлениям о клинической картине и факторах риска тяжелого течения COVID-19 у пациентов, которым рекомендовано назначение олокизумаба [3]. Продемонстрированы целесообразность назначения препарата в целевой популяции пациентов с легким и среднетяжелым течением COVID-19 и преимущества его назначения в дозе 128 мг по сравнению с дозой 64 мг с учетом тяжести течения и выраженности факторов риска у пациентов.


