В структуре инфекционной патологии детского возраста пневмонии занимают значительное место, определяя показатели здоровья, смертности и качества жизни, причем в первую очередь это касается новорожденных и детей первых месяцев жизни. От пневмонии в мире ежегодно умирают от 750 000 до 1,2 млн новорожденных, что составляет 10% от глобальной детской смертности [1]. Практика последних десятилетий не выказывает тенденции к снижению частоты врожденных, госпитальных пневмоний и пневмоний у иммунокомпрометированных детей [2]. Летальность от пневмоний среди недоношенных новорожденных колеблется от 10 до 38% [3].
Клиническая манифестация врожденной пневмониии (ВП) как правило, наступает в течение первых 3 сут жизни или 48–72 ч после рождения [2, 3]. Интранатальное инфицирование при ВП возникает при условии аспирации новорожденным околоплодных вод, инфицированных микроорганизмами, колонизирующих родовые пути матери: Staphylococcus epidermidis, Staphylococcus aureus, стрептококками группы В (SGB), Chlamydia trachomatis, грамотрицательными энтеробактериями (E. сoli, Klebsiella spp.) и др. [2]. Показано, что широкий спектр микроорганизмов за счет действия факторов патогенности влияет на показатели врожденного иммунитета на уровне слизистых оболочек дыхательных путей у новорожденных с ВП [4].
Диагностика ВП у недоношенных новорожденных представляет серьезные трудности в практической деятельности врача-неонатолога из-за того, что часть ранее описанных клинических критериев в последнее время встречается крайне редко [3]. Во многих случаях пневмония не является единственным бронхолегочным заболеванием у недоношенного новорожденного ребенка, ей часто сопутствуют респираторный дистрес-синдром новорожденного (РДСН), синдром аспирации мекония или амниотической жидкости, персистирующая легочная гипертензия (ПЛГ), функционирующий артериальный проток (ФОАП) [5]. Клиническая картина ВП у недоношенных новорожденных характеризуется появлением сразу после рождения цианоза, угнетения или возбуждения ЦНС, нарушения теплорегуляции в виде гипотермии, тахипноэ, включения в акт дыхания вспомогательной мускулатуры, приступов апноэ, пенистых выделений изо рта. Типичны ретракции грудной клетки, «хрюкающее дыхание», нарастающая вялость, тахикардия, увеличение размеров печени, падение массы тела [2]. Описанные клинические симптомы ВП неспецифичны и могут наблюдаться у недоношенных новорожденных на фоне РДСН. Показано, что патогномоничных рентгенографических признаков ВП нет [6].
В связи с тем, что незрелость и недоношенность способствуют развитию пневмонии, клинические проявления в первые часы и сутки жизни неспецифичны, направления терапии ничем не отличаются от таковой при РДСН и принципы ее применения те же [7]. Респираторная терапия, включающая искусственную вентиляцию легких (ИВЛ), является ведущим направлением лечения ВП, ее цель – достижение и поддержание адекватного газообмена и альвеолярной вентиляции, минимизация риска баротравмы и кардиогемодинамики, ликвидация десинхронизации дыхательных попыток пациента и аппаратного дыхания [2, 5]. Препаратами эмпирического выбора при ВП являются комбинация ампициллина с аминогликозидами [8], что обусловлено своеобразием этиологических факторов и клиническим манифестированием в течение первых 3 сут. жизни ребенка. По-прежнему отмечается высокая частота необоснованного назначения антимикробной терапии у критически больных новорожденных, в том числе у недоношенных детей [9].
Необходимость включения в терапию неонатальных инфекций препаратов с иммуномодулирующим действием остается предметом обсуждения. Современные подходы к иммуномодулирующей терапии включают применение различных иммунобиологических препаратов направленного действия, таких как иммуноглобулины для внутривенного введения; препараты на основе рекомбинантного интерферона (ИФН); препараты рекомбинантных цитокинов, колониестимулирующих факторов и моноклональных антител к ряду провоспалительных медиаторов иммунной системы с учетом их значимости в патогенезе заболевания [10]. Известно, что в период разгара инфекционных заболеваний дыхательной системы у детей раннего возраста значительно снижаются концентрация ИФН-α и ИФН-γ в сыворотке крови, способность к индуцированной продукции ИФН иммунокомпетентными клетками, функциональная активность натуральных киллеров [11]. Получены доказательства иммуномодулирующего действия виферона®, которое выражается в разнонаправленном изменении уровней индуцированной продукции ИФН-α и ИФН-γ лейкоцитами новорожденных с внутриутробными инфекциями в зависимости от исходного уровня продукции эндогенных ИФН [12]. В последнее время доказана целесообразность включения в комплексную терапию ВП у новорожденных препаратов ИФН [13].
ИФН проявляют противовирусную, антимикробную, антипролиферативную, иммуномодулирующую и радиопротективную активность. Малые концентрации ИФН всех классов активируют систему естественных киллерных клеток, обладающих неспецифической цитотоксичностью, что позволяет подавлять развитие инфекции на ранних этапах [14]. У детей раннего возраста с инфекционно-воспалительными заболеваниями нарушается функционирование иммунной системы и тесно связанной с ней системы ИФН, что и лежит в основе формирования заболеваний различных органов и систем. В большей степени эти нарушения присутствуют у недоношенных детей первого года жизни, в том числе с очень низкой массой тела при рождении [12]. Актуальность данного исследования обусловлена дефицитом научных данных об использовании ИФН в комплексной терапии инфекционно-воспалительных заболеваний в периоде новорожденности, в том числе ВП у недоношенных новорожденных.
Цель исследования – изучение эффективности назначения человеческого рекомбинантного ИФН-α-2b в составе комплексной терапии ВП у недоношенных новорожденных в условиях отделения реанимации и интенсивной терапии новорожденных (ОРИТН).
Материалы и методы
Исследование проводили в период с марта 2017 г. по май 2018 г. в ОРИТН ГУЗ «Клиническая больница № 5» Волгограда. В него было включено 62 новорожденных гестационного возраста 29–34 нед. с установленным в возрасте первых 3 сут. жизни диагнозом «врожденная пневмония» (код МКБ-10 P23). Диагноз выставлялся с использованием клинико-лабораторных критериев, установленных клиническими рекомендациями Российского общества неонатологов «Врожденная пневмония» и классификации неонатальных пневмоний К.А. Сотниковой [5]. После одобрения локального этического комитета и подписания информированного согласия на проведение исследования законными представителями пациентов методом конвертов пациенты были рандомизированы на 2 группы: в основную группу вошли 30 новорожденных, получавших в составе комплексной терапии ВП человеческий рекомбинантный ИФН-α-2b (виферон®, суппозитории ректальные 150 000 МЕ); группу сравнения составили 32 недоношенных ребенка, получавших только комплексную терапию ВП. Срок начала терапии вифероном® – не позднее 72 ч с момента начала заболевания, продолжительность лечения – 10 дней. Период последующего наблюдения продлился 3 мес.: клиническое обследование детей, включенных в исследование, проводили в возрасте 1 и 3 мес. жизни.
Критерии невключения: гестационный возраст до 29 и более 34 нед.; генетическая и хромосомная патология; отказ законного представителя ребенка от использования препарата; перинатальный контакт с ВИЧ-инфекцией, сифилисом, гепатитами B, С.
Критерии исключения: наличие установленных показаний к трансфузии препаратов крови и/или иммуноглобулинов, а также установленная аллергическая реакция и другие нежелательные эффекты в момент первого введения исследуемого препарата.
Для статистической обработки данных использовали пакет прикладных программ «Statistica v.9». Статистический анализ проведен с использованием непараметрических методов: двустороннего теста Фишера, теста Манна–Уитни. Количественные данные представлены в формате Ме (медиана), интеквартильный размах – LQ–UQ (25–75% процентили). Рассчитывали среднее арифметическое и стандартную ошибку (M ± m). Различия между группами по количественным параметрам оценивали с помощью критерия Стьюдента (t) и U-критерия Манна–Уитни. Различия считали статистически значимыми при р < 0,05.
Среди материнских факторов риска развития ВП у недоношенных новорожденных преобладали отягощенный акушерско-гинекологический анамнез, наличие острых и хронических инфекций во время беременности, хориоамнионит, бактериурия, дородовое излитие околоплодных вод, длительный безводный промежуток, экстрагенитальная патология матери, осложненное течение беременности (исмико-цервикальная недостаточность, преэклампсия, многоводие или маловодие, отслойка плаценты, задержка внутриутробного развития – ЗВУР) и др. Достоверных различий по частоте выявления материнских факторов риска ВП в группах не выявлено.
Всем новорожденным, включенным в исследование, проводили профилактику гипотермии в родильном зале, отсроченное пережатие или «milking» пуповины при рождении, раннее начало респираторной терапии (инвазивная респираторная терапия в родильном зале), мониторинг показателей ЧСС и SрО2 методом пульсоксиметрии, введение сурфактанта при наличии показаний [9]. Обследование и лечение новорожденных с ВП осуществляли в условиях ОРИТН с обеспечением микроклимата кувеза, ограничением сенсорной стимуляции, контролем температуры тела в зависимости от терморегуляции, постуральной поддержкой, профилактикой болевого синдрома, контролем эффективности нутритивной поддержки.
В соответствии с установленными правилами объективного осмотра новорожденного проводили клиническое обследование всех детей ежедневно в течение первых 10 дней исследования, после перевода в отделение патологии новорожденных и недоношенных детей – в возрасте 1 мес. Оценивали степень дыхательной недостаточности по шкале Сильвермана–Андерсена; степень соответствия физического развития и нервно-мышечной зрелости ребенка его гестационному возрасту; параметры физического развития [15]. Показатели антропометрии в пределах от 10 до 90 центиля оценивались как соответствующие гестационному возрасту. Отсутствовали статистически достоверные различия по гестационному возрасту, гендерному признаку, антропометрическим показателям при рождении, оценкам по шкале Апгар на 5-й мин. (p > 0,05). В табл. 1 приведена клиническая характеристика новорожденных обеих групп.

Тяжесть состояния пациентов ежедневно оценивали по неонатальной шкале оценки полиорганной недостаточности NEOMOD (Neonatal Multiple Organ Disfunction Score), которая включает 7 параметров (несостоятельность ЦНС, сердечно-сосудистой системы, органов дыхания, почек, гастроинтестинального тракта, состояние гемокоагуляции, кислотно-щелочное состояние) и предназначена для максимально объективного отражения степени и характери дисфункции органов и систем у разных групп больных в динамике заболевания [16, 17]. Осуществляли неинвазивный мониторинг основных показателей жизнеобеспечения (ЧСС, ЧД, АД, SatO2, температура тела, диурез), а также лабораторные исследования: клинический и биохимический (СРБ ) анализы крови, микробиологическое исследование крови и аспиратов из трахеи с определением чувствительности выделенной микрофлоры к антибиотикам не позднее 24 ч от момента выявления дыхательных нарушений и через 10 дней от начала лечения. Кроме того, всем новорожденным на 1-е и 10-е сутки исследования проводили микробиологические исследования содержимого трахеальных аспиратов, крови и мочи (для выявления дополнительных инфекционно-воспалительных очагов на фоне течения ВП).
У всех детей изучали биохимические анализы крови на ПКТ, КОС, газы крови, электролиты; глюкозу, билирубин; а также общий анализ мочи. Всем детям проводили ЭхоКГ, гипероксический тест, пульсоксиметрию на руках и ногах для исключения критического врожденного порока сердца (ВПС), ПЛГ и ФОАП. Для оценки кислородного статуса у детей, находившихся на ИВЛ, ежедневно рассчитывали индекс оксигенации (OI) по формуле:
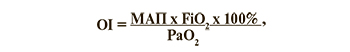
где МАП – среднее давление в дыхательных путях, см. вод. ст;
FiО2 – фракция вдыхаемого кислорода, 0,21–1,0;
РаО2 – напряжение кислорода в крови, мм рт. ст. [18].
Рентгенографию органов грудной клетки проводили всем новорожденным в течение первых 24 ч при подозрении на ВП, в дальнейшем с периодичностью 1 раз в 10–14 дней до рентгенографического разрешения, при наличии показаний – чаще.
Лечение новорожденных детей осуществляли в условиях ОРИТН, где всем пациентам дифференцированно назначали этиотропную и целенаправленную антибактериальную, противогрибковую, инфузионную терапию, парентеральное питание, вазоактивные препараты в дозировках, соответствующих утвержденным инструкциям к препаратам, а также дотацию воздушно-кислородной смеси, и/или неинвазивную ИВЛ, и/или традиционную ИВЛ, и/или высокочастотную осцилляторную ИВЛ в зависимости от медицинских показаний (табл. 2).
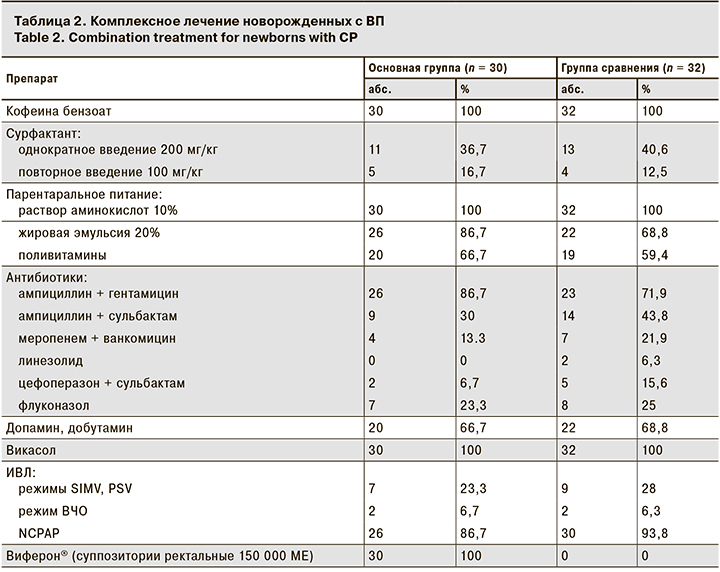
Установлено отсутствие достоверных различий между группами в составе базисной этиотропной и посиндромной терапии, в количестве новорожденных, находящихся на ИВЛ, нуждавшихся в проведении парентерального питания, введении сурфактанта, назначении нестероидных противовоспалительных препаратов (p > 0,05).
Результаты и обсуждение
У недоношенных новорожденных в основной группе выявлена более динамичная регрессия клинических проявлений ВП к 10-му дню исследования. Темпы нормализации показателей температурного гомеостаза, купирования тахикардии, снижения потребности в кислороде, нормализации дисфункций пищеварительного тракта у детей в основной группе были значимо выше, чем в группе сравнения (p < 0,05; сила связи средняя). Через 10 дней от начала лечения не выявлено достоверной разницы в динамике брадикардии, артериальной гипотензии, централизации кровообращения с нарушением перфузии кожи, олигурии, интолерантности к энтеральному питанию, судорог, геморрагического синдрома, снижения массы тела (p > 0,05).
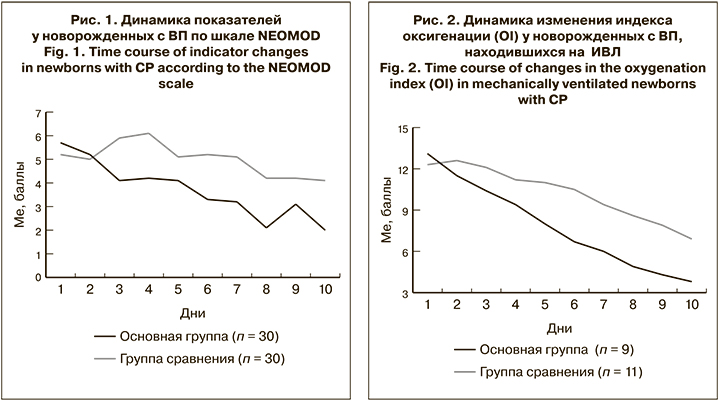
Нормализация клинических симптомов ВП у недоношенных детей основной группы подтверждается результатами динамической оценки тяжести состояния по шкале NEOMOD, средние значения которой в первые сутки исследования составили в основной группе 5,7 ± 0,7, в группе сравнения – 5,2 ± 0,9 (р > 0,05). К 10-му дню исследования оценка по шкале NEOMOD в основной группе составила 2,0 ± 0,4 балла, в группе сравнения – 4,1 ± 0,9 балла (р < 0,05). Результаты представлены на рис 1.
К 10-му дню от начала лечения у большинства детей основной группы, в отличие от группы сравнения, выявлена статистически достоверная положительная динамика лабораторных показателей: уменьшились лейкоцитоз, нейтрофилез, нейтрофильный индекс интоксикации, а также уровень ПКТ и лактата; нормализовался мочевой синдром; снизились уровни лейкоцитурии и бактериурии (p < 0,05; сила связи средняя). В то же время не обнаружено различий в динамике показателей лейкопении, нейтропении, тромбоцитопении, гликемии, протеинурии, уровня СРБ (p > 0,05).
Динамика нормализации кислородного статуса у новорожденных основной группы представлена на рис. 2. Не выявлено достоверной разницы средних значений OI на фоне ИВЛ в группах в силу малой выборки. Так, в 1-е сутки при поступлении ребенка в ОРИТН показатель OI в основной группе (n = 9) составил13,1 ± 2,6, в группе сравнения (n = 11) – 12,3 ± 1,8, а к 10-му дню от начала лечения он равнялся 3,8 ± 0,8 и 6,9 ± 1,2 соответственно (р > 0,05). Тем не менее темпы нормализации кислородного статуса в основной группе были в 2 раза выше, чем в группе сравнения.
В результате микробиологического анализа в основной группе обнаружено снижение частоты выделения микробных патогенов из трахеального аспирата, взятого в 1-е и на 10-е сутки исследования (43,7 и 13,2% соответственно; р < 0,05). В группе сравнения частота положительных высевов из трахеального аспирата в динамике не уменьшилась и составила в 1-е сутки исследования 37,5%, на 10-е сутки – 43,7% (р > 0,05).
Среди патогенов, выделенных из трахеального аспирата до начала антимикробной терапии в 1-е сутки исследования, преобладали грамположительные (Staphylococcus epidermidis, Staphylococcus aureus, Group B Streptococcus – GBS): в основной группе – 33,3%, в группе сравнения – 31,2% (р > 0,05), в то время как грамотрицательные патогены (Escherichia coli, Enterobacter aerogenes, Klebsiella spp.) выделялись несколько реже – 10 и 6,3% соответственно; р > 0,05). На 10-е сутки число положительных высевов из трахеального аспирата в группе сравнения увеличилось в 2 раза (12,5%) за счет грамотрицательных патогенов, в то время как частота выделения грамположительных патогенов не изменилась (31,2%). В основной группе отмечено снижение частоты положительных высевов в 3 раза за счет как грамположительных, так и грамотрицательных патогенов (9,9 и 3,3% соответственно). Достоверных различий в частоте выделения микробных патогенов из крови в динамике в группах не выявлено, причем представительство грамположительной микрофлоры преобладало в обеих группах.
Рост числа других (помимо ВП) микробно-воспалительных очагов отмечен у новорожденных группы сравнения к 10-му дню исследования, причем разница с основной группой была достоверно более значимая. Прежде всего, это проявилось манифестацией неонатальной инфекции мочевых путей, инфекции кожи, глаз, костей. Инфекции мочевых путей в основной группе отмечали в 3 раза реже, чем в группе сравнения. Динамика результатов микробиологических исследований мочи показала достоверные различия в группах на 10-е сутки от начала лечения: положительные результаты в основной группе были получены в 9,9% случаев, в группе сравнения – в 31,3% (р < 0,05). Таким образом, по результатам проведенных микробиологических исследований, уровень микробной нагрузки в основной группе недоношенных новорожденных с ВП был достоверно ниже, чем в группе сравнения.
Показатели эффективности комплексной терапии, включавшей ИФН-α-2b, представлены в табл. 3.
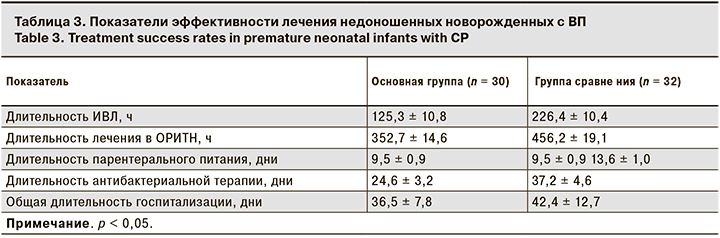
У новорожденных в основной группе сократились длительность лечения в условиях ОРИТН, продолжительность ИВЛ, назначения парентерального питания и антибактериальной терапии по сравнению с детьми в группе сравнения (р < 0,05). Не выявлено отличий в общей длительности госпитализации детей в группах исследования. Необходимость ее увеличения в большей степени была обусловлена морфофункциональной незрелостью пациентов на фоне экстремально и очень низкой массы тела при рождении, ЗВУР, манифестацией микробно-воспалительных заболеваний, а также коррелировало с наличием коморбидных состояний у недоношенных детей: РДСН, внутрижелудочковых кровоизлияний, некротизирующего энтероколита, бронхолегочной дисплазии, ретинопатии недоношенных и др.
Летальных исходов не было. Постконцептуальный возраст при выписке из неонатологического стационара составил в основной группе 35,9 ± 1,81 нед. против 36,2 ± 2,2 нед. в группе сравнения (р > 0,05). У новорожденных основной группы, в отличие от пациентов группы сравнения, по достижении возраста 1 мес. жизни достоверно реже выявляли постнатальную гипотрофию, инфекции мочевых путей (р < 0,05). Частота коморбидных состояний, таких как внутрижелудочковые кровоизлияния, протективная вентиляция легких, постгеморрагическая гидроцефалия, некротизирующий энтероколит, бронхолегочная дисплазия, ФОАП, некритические ВПС, а также бактериальный сепсис, остеомиелит и другие в группах не отличалась (р > 0,05). Примерно 2/3 недоношенных детей вскармливались грудным молоком, обогащенным фортификатором. Специальную молочную смесь для недоношенных детей получали 26,7% новорожденных основной группы и 31,3% детей группы сравнения.
Выводы
- В результате проведенного исследования получены доказательства эффективности и безопасности применения человеческого рекомбинантного ИФН-α-2b (виферон®, суппозитории ректальные 150 000 МЕ) в составе комплексной терапии ВП у недоношенных детей. У пациентов, получавших препарат, за время исследования нежелательных явлений не зафиксировано.
- У недоношенных детей, получавших в составе комплексного лечения виферон®, к 10-му дню лечения, в отличие от пациентов группы сравнения, выявлена положительная динамика клинико-лабораторных показателей, включая показатели кислородного статуса и оценку по шкале NEOMOD (р < 0,05).
- Установлено статистически значимое снижение выделения микробных патогенов из трахеального аспирата в основной группе на 10-е сутки от начала комплексной терапии, в отличие от группы сравнения. Элиминация выделенного из трахеального аспирата микробного патогена у недоношенных детей основной группы наступала в 3 раза быстрее, чем у детей в группе сравнения. Продолжительность ИВЛ, длительность лечения в условиях ОРИТН, назначения парентерального питания и антибактериальной терапии у детей в основной группе были достоверно короче, чем в группе сравнения (р < 0,05).


