Эпидемиология
Псориазом страдают более 150 млн человек, он является одним из наиболее распространенных дерматозов в мире. Это заболевание встречается повсеместно, уровень его распространенности колеблется от 0,25 до 3,5% в различных популяциях. В Европе наибольшее число больных псориазом отмечено в Норвегии (8,5%), при этом в Северной Европе среднее число больных составляет 2%, в США – 2,4%, и ежегодно регистрируется 140 000–160 000 новых случаев [1].
Псориаз распространен неравномерно в популяциях и на континентах: заболевание чаще встречается в Восточной Африке у белых эмигрантов, реже – в Западной Африке у коренных жителей – 1,2 и 2,5% соответственно. По данным исследователей, наиболее низкая заболеваемость отмечена среди индейцев Южной Америки (0,1%). Гендерных различий при псориазе не выявлено [2].
Заболевание возникает в любом возрасте, крайне редко – у детей младше 1 года. При исследовании распространенности псориаза среди детей было выявлено, что его частота в Великобритании составила 0,55% в возрастной группе 0–9 лет и 1,37% – в группе детей 10–18 лет [3]; в США – 40,8 на 100 тыс. детей в возрасте до 18 лет [4].
В развитии псориаза отмечают 2 возрастных пика: первый – в 20–40 лет, второй – в 40–60 лет.
Больные, страдающие псориазом со средним и тяжелым клиническим течением, подвержены повышенному риску смерти из-за коморбидности с сердечно-сосудистыми заболеваниями [5].
Одним из тяжелых проявлений псориаза является псориатический артрит (PSA). По разным данным, частота его у больных псориазом колеблется от 20 до 30%. Так, при обследовании 330 детей (средний возраст – 9,4 года) и 2000 взрослых (средний возраст – 48,5 года), больных псориазом, во Франции частота PSA составила 4,2% среди детей и 21% среди взрослых [6]. Исследования, выполненные в Дании с участием 13 000 больных, страдающих PSA, показали, что количество больных PSA растет. Если в 1997 г. было выявлено 7 случаев PSA в расчете на 100 тыс. населения, то в 2010 г. – уже 30 [7]. При псориазе в воспалительный процесс часто вовлекаются ногтевые пластины, что у больных PSA встречается в 70% случаев и рассматривается как патогномоничный признак PSA [8].
У больных псориазом чаще выявляют коморбидные заболевания (болезнь Крона, депрессивные расстройства, увеит, метаболический синдром), а также сердечно-сосудистую патологию, неалкогольные дистрофии печени и эректильные дисфункции [9, 10].
Лечение требует значительных финансовых затрат. По данным Американского национального фонда псориаза (National Psoriasis Foundation), в 2015 г. средняя стоимость лечения одного больного псориазом превысила 25 000 долларов, что составило более 135 млрд долларов для всех застрахованных больных, страдающих псориатической болезнью в США [1].
Частота встречаемости псориаза у ВИЧ-инфицированных различна. При обследовании в Сан-Франциско 2000 пациентов с ВИЧ-инфекцией псориаз диагностировали у 2,5% больных, в то же время в Берлине при обследовании 700 больных ВИЧ-инфекцией – у 5% [11]. На фоне ВИЧ-инфекции чаще встречаются тяжелые формы вульгарного псориаза, пустулезный псориаз, псориатическая эритродермия и PSA. На поздних стадиях ВИЧ-инфекции у больных, страдающих псориатической болезнью, отмечают воспалительные поражения глаз, поражение центральной и периферической нервной системы, признаки сердечной и почечной недостаточности [12].
Этиология и патогенез
Псориаз – мультифакторное заболевание с наследственной предрасположенностью, характеризующееся повышенной пролиферацией эпидермальных клеток, нарушением кератинизации и воспалительной реакцией в дерме, обусловленной активированными Т-лимфоцитами и синтезом провоспалительных цитокинов [13].
В развитии заболевания важную роль играет генетический фактор, вероятность его наследования оценивают в 60–90% [14].
Выделяют 9 областей PSORS (1–9), расположенных на разных генах (1q21, 3q21, 4q31) и связанных с наследованием псориаза. Помимо этого на связь с заболеванием исследуют и другие хромосомы. Наиболее ассоциированной с псориазом является аллель HLA-Cw6 (PSORS1), расположенная на 6-й хромосоме: с ней связывают развитие болезни у 10% носителей этого локуса [14].
По мнению большинства исследователей, метилирование ДНК и изменения экспрессии генов, а также связь генотипа KIR3DS1 и HLA-Bw4-80I, которая кодирует активацию рецептора CD8+ на натуральных киллерах и его лиганда, значительно повышают риск развития псориаза у ВИЧ-инфицированных больных [15, 16].
Конкордантность к псориазу у монозиготных близнецов колеблется от 35 до 75%. Этот показатель не достигает 100%, что указывает на наличие других причин развития псориатической болезни, не связанных с генетикой человека [17].
Факторами, чаще всего приводящими к манифестации и обострению заболевания, являются психоэмоциональный стресс, инфекционные болезни ротоглотки, медикаменты (β-блокаторы, соли лития, INF-α-2b), алкоголь и курение [18].
Псориаз рассматривают как аутоиммунное воспалительное заболевание (IMIDs), характеризующееся нарушением регуляции нормального иммунного ответа.
По мнению Y. Liang и соавт. [19], псориаз – это заболевание, при котором проявляются как аутоиммунные, так и аутовоспалительные реакции и клинические проявления которого зависят от баланса этих реакций. Важную роль в осуществлении этих реакций выполняют провоспалительные цитокины: в частности, при активном образовании IL-17A и др. формируются бляшки вульгарного псориаза; в другом случае при избытке IL-36/IL-1 на коже проявляется пустулезный псориаз [20, 21].
Важную роль в патогенезе псориаза играют микроРНК, регулирующие экспрессию генов посредством воздействия на мРНК приводящего к активации/дезактивации генов PSORS [22].
При гистологическом исследовании в биоптатах кожи первичного очага (его размер приблизительно 1х1 мм) отмечается выраженный отек, в верхних слоях дермы – мононуклеарные клеточные инфильтраты, в процесс вовлекаются 1–2 кожных сосочка. Над ними – выраженный спонгиоз и разрушение зернистого слоя. Вокруг капилляров в очаге отмечаются мононуклеарные инфильтраты. Эти изменения относят к первичному псориатическому очагу.
Следующий этап: зона увеличивается в размерах (приблизительно 1х1 см), растет количество макрофагов и тучных клеток в очаге с последующей их дегрануляцией, увеличивается количество Т-лимфоцитов и дендритных клеток (DC). В очагах отмечается усиление паракератоза, увеличение толщины эпидермиса, в дерме удлиняются капилляры, сохраняется или нарастает инфильтрация вокруг капилляров, усиливается межклеточный отек в эпидермисе, уменьшается количество межклеточных десмасом.
Для сформировавшейся псориатической бляшки характерно истончение надсосочкового слоя эпидермиса, значительное увеличение количества клеток в псориатической бляшке. Спонгиоз несколько уменьшается, в области базальной мембраны усиливается митотическая активность клеток. Усиливается клеточная инфильтрация вокруг капилляров, возрастает количество Т-лимфоцитов. В роговом слое в очагах паракератоза наблюдаются скопления нейтрофилов – микроабсцессы Мунро [23].
Ведущую роль в формировании псориатической бляшки выполняют кератиноциты. Они являются основными производителями провоспалительных цитокинов, факторов роста и целого ряда других медиаторов воспаления – кателицидинов, дифензинов, кальций-связывающих белков. При псориазе кератиноциты в псориатических бляшках участвуют в альтернативном механизме дифференцировки – так называемом регенеративном созревании [23].
Из клеток, связанных с первичным иммунным ответом в формирующейся псориатической бляшке, исследователи выделяют клетки Лангерганса (КЛ) (подтип DC), которые под воздействием воспалительных цитокинов завершают созревание и покидают очаг для дальнейшего привлечения в него CD4+-лимфоцитов1.
J. Seneschal и соавт. [24] отмечают роль КЛ как одного из первичных звеньев, направленных на поддержание толерантности в нормальной коже и активации защитных подмножеств Т-клеток при инфекционном воздействии.
Помимо КЛ в развитии псориатической бляшки значимую роль выполняют и другие подмножества DC. Их активация приводит к выработке ключевых псориатических цитокинов IL-12 и IL-23, причем при первичном остром псориазе ведущая роль в их выработке принадлежит плазматическим DC, при хронизации псориаза – зрелым дермальным DC и воспалительным миелоидным DC. Цитокины IL-12 и IL-23 усиливают Th1-, Th17- и Th22-ответы, активизируют выработку IL-17А, IL-22 и INF-γ, что приводит к нарушению апоптоза кератиноцитов [25].
DC являются мощными антигенпредставляющими клетками, которые модулируют антивирусный иммунный ответ. Посредствам секреции воспалительных цитокинов и интерферонов DC изменяют пролиферацию и дифференцировку Т-клеток, участвуя в иммунной дисрегуляции, встречающейся при хронической ВИЧ-инфекции. Широкое распространение DC в коже и слизистой оболочке делает их одним из первых типов клеток, которые сталкиваются с ВИЧ-инфекцией. Они являются основным фактором, способствующим активации иммунной системы и микробной транслокации через слизистую оболочку желудочно-кишечного тракта при хронической ВИЧ-инфекции [26]. Так, при длительно протекающей микробной транслокации в кишечнике наблюдается выраженный диссонанс Th17/Treg-лимфоцитов с нарушением DC секреции IL-17 и IL-23 [27], что приводит к аутоиммунным нарушениям и, в свою очередь, сказывается на клиническом течении псориаза.
Из клеток, играющих наиболее значимую роль в развитии псориаза, исследована роль популяции CD4+- и CD8+-лимфоцитов. При вульгарном псориазе одним из ключевых звеньев считается активация CD4+-лимфоцитов IL-23 с последующей активной выработкой IL-17 и привлечением в очаг регуляторных клеток для формирования хронического аутоиммунного воспаления [25].
Популяция цитотоксических CD8+-лимфоцитов, DC и других клеток достигает 80% в эпидермисе псориатических очагов, и их количество коррелирует с активностью развития псориатической болезни [26].
Важно отметить, что при отсутствии повреждения иммунокомпетентные клетки кожи не участвуют в воспалительном процессе и активизируются только после запуска каскада этих реакций [25].
CD4+- и CD8+-лимфоциты играют важную роль в формировании псориатической бляшки и патогенезе течения ВИЧ-инфекции. CD4+- и CD8+-лимфоциты кожи у больных псориазом являются основными продуцентами IL-17, активаторами Th17-ответа, формирующего аутоиммунное развитие воспаления [28]. У ВИЧ-инфицированных больных CD4+- и CD8+-лимфоциты являются одной из главных мишеней ВИЧ2.
Nagendran Prabhakaran и соавт. [29] считают объективным показателем течения псориаза у больных ВИЧ-инфецекцией нормализацию количества CD4+- и CD8+-лимфоцитов на фоне АРТ и ассоциированные с этим клинические проявления псориаза.
Таким образом, уменьшение количества CD4+- и CD8+-лимфоцитов по мере прогрессирования ВИЧ-инфекции должно приводить к уменьшению активности воспаления при псориазе, чего не происходит на практике. Последние исследования показывают, что ведущую роль играют микроРНК (miR) и метилирование коровых гистонов в хромосоме, при этом у больных отмечалась обратная корреляция метилирования и экспрессии генов (PSORS) – S100A9, SELENBP1, CARD14, KAZN и PTPN22 [30].
E. Sonkoly и соавт. [31] выявили у больных псориазом в сравнении с больными атопической экземой и здоровыми людьми наличие микроРНК miR-203 одновременно с miR-146a, miR-21 и miR-125b, что, по мнению исследователей, может рассматриваться как патогномоничный признак псориаза. Помимо этого было отмечено снижение экспрессии супрессора цитокиновых сигналов (SOCS3) под воздействием этих микроРНК и как следствие – повышение выработки IL-6, активизирующего транскрипционный фактор STAT3, приводящее к нарушению дифференцировки кератиноцитов [32].
Роль сигнальных молекул в патогенезе псориаза
Большинство изменений, возникающих в клетках кожи, обусловлено взаимодействием цитокинов, хемокинов и факторов роста с активными рецепторами клеточных мембран. Цитокины и хемокины имеют ряд общих биохимических и функциональных характеристик: разнонаправленность и взаимозаменяемость биологического действия, отсутствие антигенной специфичности, взаимодействие со специфическими клеточными рецепторами и некоторые другие2.
Одними из ключевых цитокинов в развитии вульгарного псориаза являются INF-γ, IL-1α, IL-2, IL-17A, IL-22, IL-23, онкостатин M и TNF-α [33].
Первично DC, привлеченные в очаг формирования псориатической бляшки, выделяют IL-23, относящийся к семейству цитокинов IL-12, которое играет связующую роль между врожденным и адаптивным иммунным ответом. IL-12/IL-23 воздействуют преимущественно на рецепторы CD4+- лимфоцитов, способствуя их дифференцировке по Th1-пути. При этом IL-23 преимущественно активизирует развитие CD4+-лимфоцитов типа Th17 [33].
Кроме того, CD4+-лимфоциты, находящиеся в эпидермисе, активно выделяют INF-γ, который индуцирует синтез хемокинов CXCL9, CXCL10 и CXCL11, усиливая Th1-направление иммунного ответа. Активация Th1-ответа влечет за собой активное образование Th17-клеток из наивных CD4+-лимфоцитов, усилению экспрессии IL-17, что нарушает дифференцировку кератиноцитов и приводит к их гиперплазии [33].
K.E. Nograles и соавт. [34] провели исследование кожных биоптатов у больных псориазом для выявления концентрации цитокинов в очаге воспаления и здоровой коже у одного и того же пациента, в котором приняли участие 28 больных генерализованным пустулезным псориазом Цумбуши и 12 больных вульгарным псориазом; контрольная группа – 20 человек. С помощью иммунногистохимических методов было выявлено существенное повышение уровней IL-17A, TNF-α, IL-1, IL-36 и INF-γ в очагах воспаления. При генерализованном пустулезном псориазе Цумбуши отмечена более высокая экспрессия IL-1 и IL-36 и более низкая – мРНК IL-17A и IFN-γ, чем у больных вульгарным псориазом, что указывает на роль пула цитокинов в развитии определенной формы заболевания.
Важная роль при вульгарном псориазе отводится IL-23, входящему в семейство IL-12 и имеющему общую с ним субъединицу р40. T. Iketleng и соавт. в исследовании, проводившемся в течение 24 мес. [35], выявили значительную обратную связь между количеством CD4+-лимфоцитов и уровнем IL-12p40: если в начале исследования коэффициент линейной корреляции (r) был равен -0,3710 (р < 0,0001), то через 24 мес. он составлял -0,3980 (р = 0,0032), что указывает на связь уровня IL-12p40 с более тяжелым течением псориаза у ВИЧ-инфицированных больных.
H. Guo и соавт. [36] отметили, что у таких больных вместе с вирусной нагрузкой возрастает индукция воспалительных цитокинов (IL-1, IL-1β и др.) макрофагами. A.B. Kimball и соавт. [37] в эксперименте изучали влияние IL-1β и TNF-α на изменения белков плотных контактов окклюдина, ZO-1, клаудинов 1, 4, 7, расположенных на межэпителиальных клеточных мембранах, и показали их роль в воспалении кератиноцитов на ранних этапах формирования псориатической бляшки.
Существенную роль в развитии начальных проявлений псориаза отводят INF-α и INF-γ. При исследовании их влияния на процесс апоптоза, в частности, на изменение активности цистеиновой протеазы – каспазы 8, дефицит которой приводит к возникновению воспалительных заболеваний кожи, была подтверждена роль IRF3 в формировании псориатической болезни [37].
Важное значение в развитии псориаза играет гранулоцитарно-макрофагальный колониестимулирующий фактор (EGF), регулирующий не только активность гранулоцитов и макрофагов в очаге воспаления, но и их взаимодействие с кератиноцитами через рецептор EGFR, активно влияющий на формирование воспалительного псориатического очага. М. Jost и соавт. [38] установили, что повышенное содержание EGF в коже, взаимодействуя с цитокинами TNF-α и INF-γ, приводит к формированию воспалительных очагов при псориазе и экземе.
Помимо этого в формировании псориатической бляшки значимым моментом являются изменения внутриклеточной активности и увеличение количества кальций-связывающих белков (S100A7, S100A8, S100A9 и др.), активно влияющих на ангиогенез и рост кератиноцитов в псориатической бляшке [25].
Клиника
На сегодняшний день общепринятой классификации псориаза нет.
Условно, в зависимости от формы, заболевание делят на 2 большие группы.
Наиболее распространен не пустулезный тип, включающий:
- каплевидный псориаз;
- вульгарный (бляшечный) псориаз;
- эксудативный псориаз.
Пустулезный тип включает:
- генерализованный пустулезный псориаз (тип Цумбуша);
- ограниченный пустулезный псориаз (тип Барбера);
- акродерматит хронический пустулезный Аллопо.
Согласно МКБ-10 псориаз (L40) подразделяется на:
- L40.0 Псориаз обыкновенный.
- L40.1 Генерализованный пустулезный псориаз.
- L40.2 Акродерматит стойкий [Аллопо].
- L40.3 Пустулез ладонный и подошвенный.
- L40.4 Псориаз каплевидный.
- L40.5 Псориаз артропатический.
- L40.8 Другой псориаз (инверсный псориаз).
- L40.9 Псориаз неуточненный.
Диагноз заболевания выставляют на основании специфической клинической картины, наличия «псориатической триады»:
- Феномен стеаринового пятна.
- Феномен терминальной пленки.
- Феномен «кровяной росы».
Патогномоничной гистологической картины для псориаза не выявлено.
В течении заболевания выделяют несколько периодов: первый – обострение заболевания (прогрессивная стадия), второй – стабилизация процесса (стационарная стадия), третий – стихание заболевания (регресс) и отдельно – период ремиссии.
По периодам обострения выделяют зимнюю (обострение в холодный период), летнюю (обострение, связанное в основном с УФ-воздействием; чаще встречается летом) и смешанную формы.
Для объективной оценки тяжести течения псориаза используют специальные индексы. Чаще всего – индекс тяжести поражения псориазом PASI (Psoriasis Area and Severity Index). С его помощью оценивают характер и степень кожных изменений (краснота, зуд, отек, гиперемия, шелушение) и площадь поражения псориазом по шкале от 0 (нет болезни) до 72 (максимальные кожные проявления). Индекс вычисляют по определенной формуле и используют в основном для научных целей. Для определения качества проводимой терапии у больных псориазом используют индекс PASI75, означающий улучшение относительно первоначального состояния на 75% .
Второй индекс – BSA (Body Surfase Area), где за основу взята площадь ладони человека в 1%. При суммарной площади поражения тела менее 3% (BSA < 3%) говорят о легком течении заболевания, от 3 до 9% – о течении средней тяжести, более 10% – о тяжелом течении.
Третий индекс DLQI (Dermatology Life Quality Index) – дерматологический индекс качества жизни – позволяет оценить степень влияния заболевания на качество жизни больного. Определяется с помощью протокола, содержащего 10 вопросов; значение индекса варьирует от 0 до 30 баллов [39].
Для вульгарного псориаза характерно наличие на коже папулезных элементов розово-красного цвета с четкими границами, склонных к образованию бляшек различной формы и размера, покрытых серебристо-белыми чешуйками. Высыпания чаще локализуются на коже головы, по наружной поверхности верхних и нижних конечностей и на туловище. В период обострения до 60% больных отмечают кожный зуд разной интенсивности. У больных псориазом с коморбидными заболеваниями чаще отмечается эксудативный псориаз: псориатическая бляшка пропитана экссудатом, и «псориатическая триада» зачастую не определяется или является сомнительной.
В случаях, когда вульгарный псориаз располагается на себорейных зонах (голова, грудь, спина), говорят о себорейном псориазе.
Иногда после перенесенной ангины у реконвалесцентов может наблюдаться первичное проявление псориаза. Эта форма заболевания чаще регестрируется у детей и подростков. При ней на коже видны множественные мелкие папулы ярко-красного цвета с умеренным шелушением и небольшой инфильтрацией. При повторном обострении заболевания мелкие бляшки могут объединяться и переходить в вульгарный (бляшечный) псориаз.
По разным данным, приблизительно у 10% больных диагностируют пустулезый псориаз. Выделяют 2 его формы: острый генерализованный пустулезный псориаз (тип Цумбуша) и хронический ладонно-подошвенный (тип Барбера). При этих формах заболевания (чаще при генерализованой форме) могут выявляться изменения со стороны крови. При посевах, взятых из пустул в период обострения заболевания, у больных отмечают отсутствие роста бактерий. Для генерализованного пустулезного псориаза (тип Цумбуша) характерно тяжелое течение с общими симптомами и лихорадкой
Пустулезный ладонно-подошвенный псориаз (тип Барбера) встречается несколько чаще, чем генерализованные формы. Кожные изменения располагаются в области ладоней (по краям ладони) и на коже стоп, в области свода.
Одной из самых тяжелых форм вульгарного псориаза является эритродермическая, возникающая в результате обострения псориаза под влиянием раздражающих факторов или вследствие неверного лечения. В этом случае поражается до 90% поверхности кожи, она становятся ярко красной, с выраженной инфильтрацией, при пальпации горячая на ощупь, покрыта чешуйками разной величины. Больные отмечают выраженный зуд и стягивание кожи, одновременно ухудшается общее состояние больного: температура тела повышается до 40 °С, появляются выраженные озноб и слабость, снижается аппетит, уменьшается потоотделение. При длительном течении псориатической эритродермии может происходить тотальное выпадение волос и ногтей. У 30% пациентов с псориатической болезнью наблюдается PSА, который может возникать одновременно с кожными высыпаниями, но встречается и без них. Отмечают прямую корреляцию между обострением PSА и прогрессированием кожных высыпаний.
В 70% случаев при PSА отмечается поражение ногтевых пластин. У больных на ногтях появляются точечные углубления, напоминающие поверхность наперстка (симптом «наперстка»). Под ногтевыми пластинами появляются желто-бурые пятна (симптом «масляного пятна»). Иногда встречаются утолщение и деформация ногтевых пластин – онихогрифоз [13].
На фоне ВИЧ-инфекции чаще встречаются распространенные формы вульгарного псориаза, пустулезный псориаз, псориатическая эритродермия и PSА. На поздних стадиях ВИЧ-инфекции у больных, страдающих псориатической болезнью, наблюдаются воспалительные поражения глаз, центральной и периферической нервной системы, признаки сердечной и почечной недостаточности.
Диагностика ВИЧ-ассоциированного псориаза зачастую осложнена тем, что при некоторых дерматозах (синдром Рейтера, фульминантная эритродермия и др.) изменения кожи у больных ВИЧ-инфекцией могут напоминать псориаз [12, 40].
У ВИЧ-инфицированных больных псориаз протекает тяжелее, имеет более разнообразную клиническую картину, плохо поддается стандартной терапии (рис. 1–3, см. на вклейке), поэтому исследования в этой области очень актуальны [12, 41].
Лечение
Псориаз – хроническое, медленно прогрессирующее заболевание. Лечение больных псориазом рекомендуется при средних и тяжелых формах заболевания.
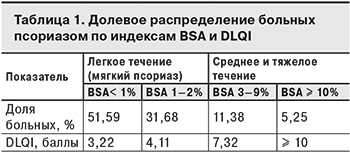 Для исследования распределения псориаза по тяжести течения на основании данных Национального обследования здоровья и питания (NHANES) в США за 2003–2004 гг. S.K. Kurd и соавт. [42] изучили 5 млн историй болезни пациентов с псориазом. Результаты представлены в табл. 1. Согласно им, 16,63% больных имеющих среднее и тяжелое течение псориаза, нуждаются в терапии.
Для исследования распределения псориаза по тяжести течения на основании данных Национального обследования здоровья и питания (NHANES) в США за 2003–2004 гг. S.K. Kurd и соавт. [42] изучили 5 млн историй болезни пациентов с псориазом. Результаты представлены в табл. 1. Согласно им, 16,63% больных имеющих среднее и тяжелое течение псориаза, нуждаются в терапии.
Кроме этого, в соответствии с решением Европейского консенсуса дерматологов 2010 г. (Дельфийский консенсус) показаниями для назначения лечения являются: наличие поражений видимых участков кожи; повреждение более 1% кожи головы; наличие высыпаний на гениталиях; вовлечение в процесс кожи стоп и кистей; кожный зуд и изменение 2 и более ногтевых пластин, а так же наличие резистентных бляшек. Терапия также рекомендуется в тех случаях, когда DLQI ≥ 10, даже если показатели BSA и PASI незначительны [43].
В терапии псориаза применяют так называемый трехступенчатый подход: при легком течении заболевания и ограниченой площади поражения используют топическую, мазевую терапию; по мере утяжеления заболевания и увеличения площади поражения добавляют физиотерапевтические методы; при тяжелом течении заболевания (BSA и PASI ≥ 10%) проводят системную терапию [44].
При назначении местной терапии необходимо учитывать стадию заболевания. В период обострения нельзя применять раздражающие препараты. Предпочтительны кератопластические и противовоспалительные средства, например, комбинированный препарат кальципотриол/бетаметазон; мази, содержащие 0,5–2% салициловой кислоты; кортикостероидные мази или крем, содержащий 0,2% активированного пиритиона цинка [44].
На стадии стихания обострения используют кальципотриол, кератолитические мази или пасты, содержащие 3–5% салициловой кислоты, 10–30% нафталана и др. При наличии кожного зуда используют лечебные ванны с растительными добавками, затем – увлажняющие, смягчающие кремы.
Физиотерапия при среднетяжелом течении псориаза включает 2 основных метода: PUVA-терапию – длинноволновое (от 320 до 400 нм) УФ-излучение и эксимерный лазер (308 нм). Можно использовать и другие физиотерапевтические методы для нормализации работы органов и систем больного3.
Выбор системной терапии определяется формой и тяжестью заболевания и наличием сопутствующих заболеваний. Чаще всего применяют метотрексат и циклоспорин. Метотрексат применяют 1–2 раза в неделю суммарно от 10 до 30 мг в сочетании с приемом фолиевой кислоты.
Циклоспорин назначают по 2,5–5мг/кг в сутки непродолжительным курсом до 12 нед. Прием обоих препаратов требует постоянного контроля из-за возможного возникновения побочных эффектов.
Для лечения тяжелых или резистентных форм псориаза используют системные ретиноиды, в частности, ацитретин в суточной дозе у взрослых 25–75 мг на протяжении 8–12 нед. Прием этих препаратов также требует контроля функции печени.
В настоящее время активно используют препараты для селективного внутриклеточного ингибирования сигнальных путей, в частности, апремиласт (блокатор фосфодиэстразы 4), который с 2014 г. начали применять в США для лечения тяжелых форм псориаза. Этот препарат имеет меньше противопоказаний, чем метотрексат, циклоспорин и системные биологические препараты [45]. Кроме того, для иммуносупрессивной терапии у больных с тяжелыми формами псориаза и PSA используют биологические препараты, перечень которых представлен в табл. 2.

Терапия псориаза у ВИЧ-инфицированных больных проводится обязательно с учетом основного заболевания. При легких или умеренных формах псориаза рекомендуют мазевое лечение, при умеренных и тяжелых формах назначают фототерапию в сочетании с препаратами первой линии. Пероральные ретиноиды могут быть использованы в качестве второй линии терапии. При более тяжелых формах заболевания можно использовать циклоспорин, метотрексат, гидроксимочевину и ингибиторы TNF-α, но с осторожностью из-за их иммуносупресивного действия. Вопрос назначения лекарственных препаратов в каждом конкретном случае решается индивидуально [44].


